Silver nanoparticles induce neurotoxicity in a human embryonic stem cell-derived neuron and astrocyte network
- PMID: 29334833
- PMCID: PMC6172039
- DOI: 10.1080/17435390.2018.1425497
Silver nanoparticles induce neurotoxicity in a human embryonic stem cell-derived neuron and astrocyte network
Abstract
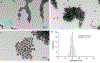
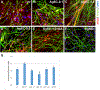
Silver nanoparticles (AgNPs) are among the most extensively used nanoparticles and are found in a variety of products. This ubiquity leads to inevitable exposure to these particles in everyday life. However, the effects of AgNPs on neuron and astrocyte networks are still largely unknown. In this study, we used neurons and astrocytes derived from human embryonic stem cells as a cellular model to study the neurotoxicity that is induced by citrate-coated AgNPs (AgSCs). Immunostaining with the astrocyte and neuron markers, glial fibrillary acidic protein and microtubule-associated protein-2 (MAP2), respectively, showed that exposure to AgSCs at the concentration of 0.1 µg/mL increased the astrocyte/neuron ratio. In contrast, a higher concentration of AgSCs (5.0 µg/ml) significantly changed the morphology of astrocytes. These results suggest that astrocytes are sensitive to AgSC exposure and that low concentrations of AgSCs promote astrogenesis. Furthermore, our results showed that AgSCs reduced neurite outgrowth, decreased the expression of postsynaptic density protein 95 and synaptophysin, and induced neurodegeneration in a concentration-dependent manner. Our findings additionally suggest that the expression and phosphorylation status of MAP2 isoforms, as modulated by the activation of the Akt/glycogen synthase kinase-3/caspase-3 signaling pathway, may play an important role in AgSC-mediated neurotoxicity. We also found that AgNO3 exposure only slightly reduced neurite outgrowth and had little effect on MAP2 expression, suggesting that AgSCs and AgNO3 have different neuronal toxicity mechanisms. In addition, most of these effects were reduced when the cell culture was co-treated with AgSCs and the antioxidant ascorbic acid, which implies that oxidative stress is the major cause of AgSC-mediated astrocytic/neuronal toxicity and that antioxidants may have a neuroprotective effect.
Keywords: Citrate-coated silver nanoparticles; human embryonic stem cells; nanotoxicity; neurons and astrocytes; neurotoxicity.
Conflict of interest statement
DECLARATION OF INTEREST
The authors report no conflicts of interest.
Figures



References
-
- Arora S, Jain J, Rajwade J,Paknikar K. 2008. Cellular responses induced by silver nanoparticles: in vitro studies. Toxicology letters 179: 93–100. - PubMed
-
- Begum AN, Aguilar JS, Elias L,Hong Y. 2016. Silver nanoparticles exhibit coating and dose-dependent neurotoxicity in glutamatergic neurons derived from human embryonic stem cells. NeuroToxicology 57: 45–53. - PubMed
-
- Biosciences B. Caspase-3 Activation-An Indicator of Apoptosis in Image-Based Assays. 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
