Performance evaluation of existing immunoassays for Clonorchis sinensis infection in China
- PMID: 29334990
- PMCID: PMC5769360
- DOI: 10.1186/s13071-018-2612-3
Performance evaluation of existing immunoassays for Clonorchis sinensis infection in China
Abstract
Background: Clonorchiasis ranks among the most important food-borne parasitic diseases in China. However, due to low compliance to traditional fecal examination techniques in the general population and medical personnel, immunodiagnosis is expected. This study evaluated, in parallel, the performance of four immunodiagnostic kits detecting clonorchiasis in China.
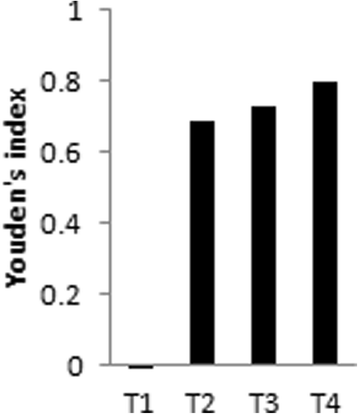
Results: A bank with 475 sera was established in this study. Except for the low performance of the kit detecting IgM, the other three kits detecting IgG showed sensitivities ranging from 81.51% (194/238) to 99.16% (236/238). Higher sensitivity was presented in heavy infection intensity [89.47% (68/76) to 100% (76/76)]. Among the four kits, the overall specificity varied from 73.42% (174/237) to 87.34% (207/237). It was observed that the specificity was lower in the sera of the participants living in clonorchiasis-endemic areas but without any parasite infection [67.5% (81/120) to 90% (108/120)], as compared to those from the non-endemic area [94% (47/50) to 98% (49/50)]. The cross-reaction rate varied from 14.93% (10/67) to 31.34% (21/67). Youden's index was -0.022, 0.689, 0.726, and 0.802 for kits T1, T2, T3 and T4, respectively. Repeatability was high in all four kits.
Conclusions: Three immunodiagnosis kits targeting IgG antibody had high performance on detecting chronic Clonorchis sinensis infection, but that detecting IgM antibody had not. The kits detecting IgG antibody also showed high sensitivity in heavy infection intensity. Research on immunological diagnosis of clonorchiasis is expected to be strengthened to improve the sensitivity in light infection and specificity.
Keywords: Clonorchiasis; Clonorchis sinensis; Evaluation; Immunodiagnosis; Sensitivity; Specificity; Youden’s index.
Conflict of interest statement
Ethics approval and consent to participate
The experimental methods involving sera used in this study were in accordance with the approved guidelines and regulations of the Key Laboratory of Parasite and Vector Biology, Ministry of Health. The collection of sera and study protocol in the endemic area were embedded in a control pilot. Ethical clearance of this pilot had been granted approval by the Ethics Committee of the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention in Shanghai, China (permit number 2011-005). The objectives, procedures, and potential risks were orally explained to all participants. A written consent form was also obtained from each participant with the participant’s signature or that of a proxy.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical