Sex differences in the late first trimester human placenta transcriptome
- PMID: 29335024
- PMCID: PMC5769539
- DOI: 10.1186/s13293-018-0165-y
Sex differences in the late first trimester human placenta transcriptome
Abstract
Background: Development of the placenta during the late first trimester is critical to ensure normal growth and development of the fetus. Developmental differences in this window such as sex-specific variation are implicated in later placental disease states, yet gene expression at this time is poorly understood.
Methods: RNA-sequencing was performed to characterize the transcriptome of 39 first trimester human placentas using chorionic villi following genetic testing (17 females, 22 males). Gene enrichment analysis was performed to find enriched canonical pathways and gene ontologies in the first trimester. DESeq2 was used to find sexually dimorphic gene expression. Patient demographics were analyzed for sex differences in fetal weight at time of chorionic villus sampling and birth.
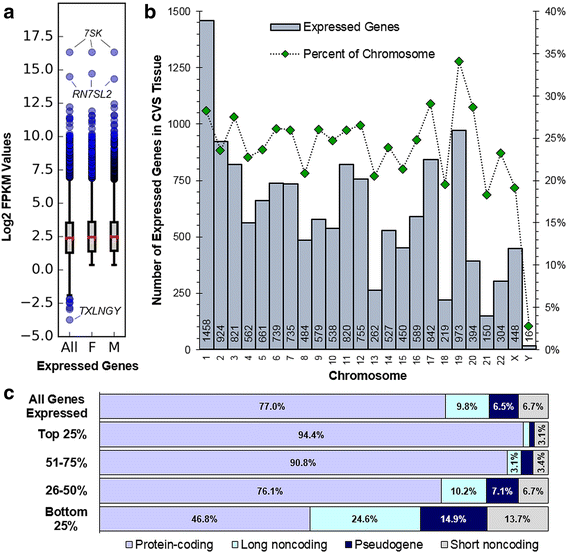
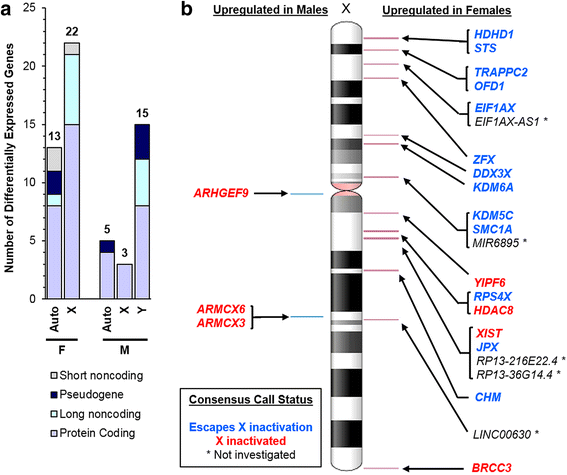
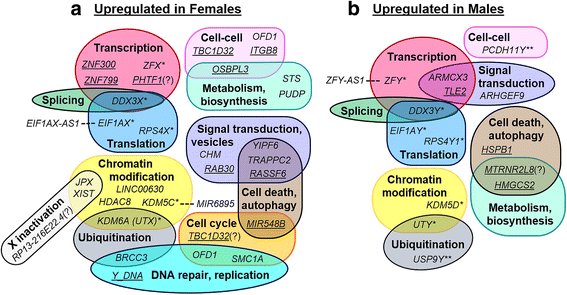
Results: RNA-sequencing analyses detected 14,250 expressed genes, with chromosome 19 contributing the greatest proportion (973/2852, 34.1% of chromosome 19 genes) and Y chromosome contributing the least (16/568, 2.8%). Several placenta-enriched genes as well as histone-coding genes were identified to be unique to the first trimester and common to both sexes. Further, we identified 58 genes with significantly different expression between males and females: 25 X-linked, 15 Y-linked, and 18 autosomal genes. Genes that escape X inactivation were highly represented (59.1%) among X-linked genes upregulated in females. Many genes differentially expressed by sex consisted of X/Y gene pairs, suggesting that dosage compensation plays a role in sex differences. These X/Y pairs had roles in parallel, ancient canonical pathways important for eukaryotic cell growth and survival: chromatin modification, transcription, splicing, and translation.
Conclusions: This study is the first characterization of the late first trimester placenta transcriptome, highlighting similarities and differences among the sexes in ongoing human pregnancies resulting in live births. Sexual dimorphism may contribute to pregnancy outcomes, including fetal growth and birth weight, which was seen in our cohort, with males significantly heavier than females at birth. This transcriptome provides a basis for development of early diagnostic tests of placental function that can indicate overall pregnancy heath, fetal-maternal health, and long-term adult health.
Keywords: Chorionic villus sampling; First trimester placenta; Pregnancy; RNA-sequencing; Sex differences.
Conflict of interest statement
Ethics approval and consent to participate
Patients were recruited with informed written consent. All protocols were performed in accordance with the institutional review board’s guidelines at the Cedars-Sinai Medical Center under IRB Protocols Pro00006806 (prenatal repository) and Pro00008600 (differential gene expression of early placenta).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

