SPARC expression is associated with hepatic injury in rodents and humans with non-alcoholic fatty liver disease
- PMID: 29335425
- PMCID: PMC5768809
- DOI: 10.1038/s41598-017-18981-9
SPARC expression is associated with hepatic injury in rodents and humans with non-alcoholic fatty liver disease
Abstract
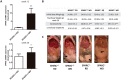
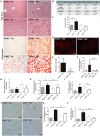
Mechanisms that control progression from simple steatosis to steato-hepatitis and fibrosis in patients with non-alcoholic fatty liver disease (NAFLD) are unknown. SPARC, a secreted matricellular protein, is over-expressed in the liver under chronic injury. Contribution of SPARC accumulation to disease severity is largely unknown in NAFLD. We assessed the hypothesis that SPARC is increased in livers with more necrosis and inflammation and could be associated with more fibrosis. qrt-PCR, immunohistochemistry, and ELISA were employed to localize and quantify changes in SPARC in 62 morbidly obese patients with NAFLD/NASH and in a mouse model of diet-induced-NASH. Results were correlated with the severity of NAFLD/NASH. In obese patients 2 subgroups were identified with either high SPARC expression (n = 16) or low SPARC expression (n = 46) in the liver, with a cutoff of 1.2 fold expression. High expression of SPARC paralleled hepatocellular damage and increased mRNA expression of pro-fibrogenic factors in the liver. In line with these findings, in the NASH animal model SPARC knockout mice were protected from inflammatory injury, and showed less inflammation and fibrosis. Hepatic SPARC expression is associated with liver injury and fibrogenic processes in NAFLD. SPARC has potential as preventive or therapeutic target in NAFLD patients.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Angulo, P., Keach, J. C., Batts, K. P. & Lindor, K. D. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology30, 1356–1362, S0270913999005017 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

