Synaptic localisation of SRF coactivators, MKL1 and MKL2, and their role in dendritic spine morphology
- PMID: 29335431
- PMCID: PMC5768758
- DOI: 10.1038/s41598-017-18905-7
Synaptic localisation of SRF coactivators, MKL1 and MKL2, and their role in dendritic spine morphology
Abstract
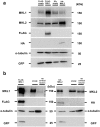
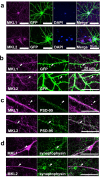
The megakaryoblastic leukaemia (MKL) family are serum response factor (SRF) coactivators, which are highly expressed in the brain. Accordingly, MKL plays important roles in dendritic morphology, neuronal migration, and brain development. Further, nucleotide substitutions in the MKL1 and MKL2 genes are found in patients with schizophrenia and autism spectrum disorder, respectively. Thus, studies on the precise synaptic localisation and function of MKL in neurons are warranted. In this study, we generated and tested new antibodies that specifically recognise endogenously expressed MKL1 and MKL2 proteins in neurons. Using these reagents, we biochemically and immunocytochemically show that MKL1 and MKL2 are localised at synapses. Furthermore, shRNA experiments revealed that postsynaptic deletion of MKL1 or MKL2 reduced the percentage of mushroom- or stubby-type spines in cultured neurons. Taken together, our findings suggest that MKL1 and MKL2 are present at synapses and involved in dendritic spine maturation. This study may, at least in part, contribute to better understanding of the molecular mechanisms underlying MKL-mediated synaptic plasticity and neurological disorders.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

