Evidence of a cubic iron sub-lattice in t-CuFe2O4 demonstrated by X-ray Absorption Fine Structure
- PMID: 29335500
- PMCID: PMC5768695
- DOI: 10.1038/s41598-017-19045-8
Evidence of a cubic iron sub-lattice in t-CuFe2O4 demonstrated by X-ray Absorption Fine Structure
Abstract
Copper ferrite, belonging to the wide and technologically relevant class of spinel ferrites, was grown in the form of t-CuFe2O4 nanocrystals within a porous matrix of silica in the form of either an aerogel or a xerogel, and compared to a bulk sample. Extended X-ray absorption fine structure (EXAFS) spectroscopy revealed the presence of two different sub-lattices within the crystal structure of t-CuFe2O4, one tetragonal and one cubic, defined by the Cu2+ and Fe3+ ions respectively. Our investigation provides evidence that the Jahn-Teller distortion, which occurs on the Cu2+ ions located in octahedral sites, does not affect the coordination geometry of the Fe3+ ions, regardless of their location in octahedral or tetrahedral sites.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




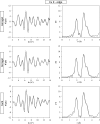
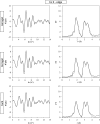
References
-
- Kefeni KK, Msagati TA, Mamba BB. Ferrite nanoparticles: synthesis, characterisation and applications in electronic device. Mater. Sci. Eng.: B. 2017;215:37–55. doi: 10.1016/j.mseb.2016.11.002. - DOI
-
- Qu Y, et al. The effect of reaction temperature on the particle size, structure and magnetic properties of co-precipitated CoFe2O4 nanoparticles. Mater. Lett. 2006;60:3548–3552. doi: 10.1016/j.matlet.2006.03.055. - DOI
-
- Tiano AL, et al. Correlating size and composition-dependent effects with magnetic, Mössbauer, and pair distribution function measurements in a family of catalytically active ferrite nanoparticles. Chem. Mater. 2015;27:3572–3592. doi: 10.1021/acs.chemmater.5b00767. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

