A versatile MOF-based trap for heavy metal ion capture and dispersion
- PMID: 29335517
- PMCID: PMC5768720
- DOI: 10.1038/s41467-017-02600-2
A versatile MOF-based trap for heavy metal ion capture and dispersion
Abstract
Current technologies for removing heavy metal ions are typically metal ion specific. Herein we report the development of a broad-spectrum heavy metal ion trap by incorporation of ethylenediaminetetraacetic acid into a robust metal-organic framework. The capture experiments for a total of 22 heavy metal ions, covering hard, soft, and borderline Lewis metal ions, show that the trap is very effective, with removal efficiencies of >99% for single-component adsorption, multi-component adsorption, or in breakthrough processes. The material can also serve as a host for metal ion loading with arbitrary selections of metal ion amounts/types with a controllable uptake ratio to prepare well-dispersed single or multiple metal catalysts. This is supported by the excellent performance of the prepared Pd2+-loaded composite toward the Suzuki coupling reaction. This work proposes a versatile heavy metal ion trap that may find applications in the fields of separation and catalysis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
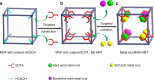
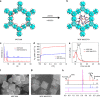

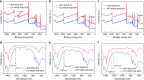
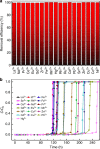

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

