Sphingadienes show therapeutic efficacy in neuroblastoma in vitro and in vivo by targeting the AKT signaling pathway
- PMID: 29335887
- PMCID: PMC6047934
- DOI: 10.1007/s10637-017-0558-5
Sphingadienes show therapeutic efficacy in neuroblastoma in vitro and in vivo by targeting the AKT signaling pathway
Erratum in
-
Correction to: Sphingadienes show therapeutic efficacy in neuroblastoma in vitro and in vivo by targeting the AKT signaling pathway.Invest New Drugs. 2019 Dec;37(6):1309. doi: 10.1007/s10637-019-00772-w. Invest New Drugs. 2019. PMID: 31032525
Abstract
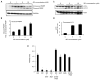
Neuroblastoma is a childhood malignancy that accounts for approximately 15% of childhood cancer deaths. Only 20-35% of children with metastatic neuroblastoma survive with standard therapy. Identification of more effective therapies is essential to improving the outcome of children with high-stage disease. Sphingadienes (SD) are growth-inhibitory sphingolipids found in natural sources including soy. They exhibit chemopreventive activity in mouse models of colon cancer, where they mediate cytotoxicity by inhibiting key pro-carcinogenic signaling pathways. In this study, the effect of SD on neuroblastoma was analyzed. Low micromolar concentrations of SD were cytotoxic to transformed and primary neuroblastoma cells independently of N-Myc amplification status. SD induced both caspase-dependent apoptosis and autophagy in neuroblastoma cells. However, only inhibition of caspase-dependent apoptosis protected neuroblastoma cells from SD-mediated cytotoxicity. SD also inhibited AKT activation in neuroblastoma cells as shown by reduced phosphorylated AKT levels. Pre-treatment with insulin attenuated SD-mediated cytotoxicity in vitro. SD-loaded nanoparticles (NP) administered parenterally to immunodeficient mice carrying neuroblastoma xenografts resulted in cytotoxic levels of SD in the circulation and significantly reduced tumor growth compared to vehicle-treated controls. Analysis of tumor extracts demonstrated reduced AKT activation in tumors of mice treated with SD-NP compared to controls treated with empty NP. Our findings indicate SD are novel potential chemotherapeutic agents that promote neuroblastoma cell death and reduce tumorigenicity in vivo.
Keywords: AKT; Nanoparticle; Neuroblastoma; PI3K; Sphingadienes; Sphingolipids.
Figures
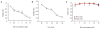



References
-
- Ries LA, et al. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. Bethesda, MD: National Cancer Institute; 1999. SEER Program, NIH Pub. No. 99 4649 192.
-
- Young JL, Jr, Miller RW. Incidence of malignant tumors in U. S. children. Journal of Pediatrics. 1975;86(2):254–8. - PubMed
-
- Frappaz D, et al. LMCE3 treatment strategy: results in 99 consecutively diagnosed stage 4 neuroblastomas in children older than 1 year at diagnosis. Journal of Clinical Oncology. 2000;18(3):468–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

