Increased Mortality Rates With Prolonged Corticosteroid Therapy When Compared With Antitumor Necrosis Factor-α-Directed Therapy for Inflammatory Bowel Disease
- PMID: 29336432
- PMCID: PMC5886050
- DOI: 10.1038/ajg.2017.479
Increased Mortality Rates With Prolonged Corticosteroid Therapy When Compared With Antitumor Necrosis Factor-α-Directed Therapy for Inflammatory Bowel Disease
Abstract
Objectives: Crohn's disease (CD) and ulcerative colitis (UC) are inflammatory bowel diseases (IBD) that compromise quality of life and may increase mortality. This study compared the mortality risk with prolonged corticosteroid use vs. antitumor necrosis factor-α (anti-TNF) drugs in IBD.
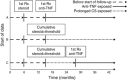
Methods: A retrospective cohort study was conducted among Medicaid and Medicare beneficiaries from 2001 to 2013 with IBD prescribed either >3,000 mg of prednisone or equivalent within a 12-month period or new initiation of anti-TNF therapy, each treated as time-updating exposures. The primary outcome was all-cause mortality. Secondary outcomes included common causes of death. Marginal structural models were used to determine odds ratios (ORs) and 95% confidence intervals (CIs) for anti-TNF use relative to corticosteroids.
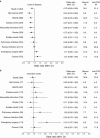
Results: Among patients with CD, 7,694 entered the cohort as prolonged corticosteroid users and 1,879 as new anti-TNF users. Among patients with UC, 3,224 and 459 entered the cohort as prolonged CS users and new anti-TNF users, respectively. The risk of death was statistically significantly lower in patients treated with anti-TNF therapy for CD (21.4 vs. 30.1 per 1,000 person-years, OR 0.78, 0.65-0.93) but not for UC (23.0 vs. 30.9 per 1,000 person-years, OR 0.87, 0.63-1.22). Among the CD cohort, anti-TNF therapy was also associated with lower rates of major adverse cardiovascular events (OR 0.68, 0.55-0.85) and hip fracture (OR 0.54, 0.34-0.83).
Conclusions: Compared with prolonged corticosteroid exposure, anti-TNF drug use was associated with reduced mortality in patients with CD that may be explained by lower rates of major adverse cardiovascular events and hip fracture.
Conflict of interest statement
Figures


Comment in
-
Past Time for Doctors to Lessen their Dependence on Corticosteroids in the Treatment of IBD.Am J Gastroenterol. 2018 Mar;113(3):418-420. doi: 10.1038/ajg.2018.9. Am J Gastroenterol. 2018. PMID: 29535444
References
-
- Herrinton LJ, Liu L, Lafata JE et al. Estimation of the period prevalence of inflammatory bowel disease among nine health plans using computerized diagnoses and outpatient pharmacy dispensings. Inflamm Bowel Dis 2007;13:451–461. - PubMed
-
- Herrinton LJ, Liu L, Lewis JD et al. Incidence and prevalence of inflammatory bowel disease in a Northern California managed care organization, 1996-2002. Am J Gastroenterol 2008;103:1998–2006. - PubMed
-
- Loftus EV Jr, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn's disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther 2002;16:51–60. - PubMed
-
- Loftus EV Jr, Silverstein MD, Sandborn WJ et al. Crohn's disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology 1998;114:1161–1168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

