Challenges in the Conversion of Manual Processes to Machine-Assisted Syntheses: Activation of Thioglycoside Donors with Aryl(trifluoroethyl)iodonium Triflimide
- PMID: 29336575
- PMCID: PMC5898625
- DOI: 10.1021/acs.orglett.7b03940
Challenges in the Conversion of Manual Processes to Machine-Assisted Syntheses: Activation of Thioglycoside Donors with Aryl(trifluoroethyl)iodonium Triflimide
Abstract
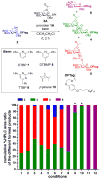
The steps needed to adapt a stable iodonium promoter for use in automated fluorous-assisted solution-phase oligosaccharide synthesis are described. Direct adaptation of the originally reported batch procedure resulted in the formation of an orthoester or protecting group transfer to the glycosyl acceptor. Fortunately, the addition of inexpensive β-pinene as an acid scavenger avoided both of these side reactions. The utility of this newly developed protocol was applied to the automated solution-phase synthesis of a β-glucan fragment.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Plante OJ, Palmacci ER, Seeberger PH. Science. 2001;291:1523–1527. - PubMed
- Plante OJ, Palmacci ER, Seeberger PH. Adv Carbohydr Chem Biochem. 2003;58:35–54. - PubMed
- Hahm HS, Schlegel MK, Hurevich M, Eller S, Schuhmacher F, Hofmann J, Pagel K, Seeberger PH. Proc Natl Acad Sci U S A. 2017;114:E3385–E3389. - PMC - PubMed
- Geertbeda A, van Mechelen J, Meeuwenoord N, Overkleeft HS, van der Marel GA, Codee JDCJ. Org Chem. 2017;82:12992–13002. - PMC - PubMed
- Komba S, Terauchi T, Machida S. J Appl Glycosci. 2009;56:193–206.
-
- Bauer J, Brandenburg K, Zahringer U, Rademann J. Chem -Eur J. 2006;12:7116–7124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

