LXR/ApoE Activation Restricts Innate Immune Suppression in Cancer
- PMID: 29336888
- PMCID: PMC5846344
- DOI: 10.1016/j.cell.2017.12.026
LXR/ApoE Activation Restricts Innate Immune Suppression in Cancer
Abstract
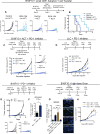
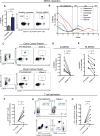
Therapeutic harnessing of adaptive immunity via checkpoint inhibition has transformed the treatment of many cancers. Despite unprecedented long-term responses, most patients do not respond to these therapies. Immunotherapy non-responders often harbor high levels of circulating myeloid-derived suppressor cells (MDSCs)-an immunosuppressive innate cell population. Through genetic and pharmacological approaches, we uncovered a pathway governing MDSC abundance in multiple cancer types. Therapeutic liver-X nuclear receptor (LXR) agonism reduced MDSC abundance in murine models and in patients treated in a first-in-human dose escalation phase 1 trial. MDSC depletion was associated with activation of cytotoxic T lymphocyte (CTL) responses in mice and patients. The LXR transcriptional target ApoE mediated these effects in mice, where LXR/ApoE activation therapy elicited robust anti-tumor responses and also enhanced T cell activation during various immune-based therapies. We implicate the LXR/ApoE axis in the regulation of innate immune suppression and as a target for enhancing the efficacy of cancer immunotherapy in patients.
Keywords: ApoE; LRP8; LXR; MDSC; cancer; clinical trial; immune therapy; myeloid; nuclear hormone receptor; tumor immunology.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
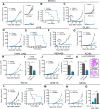
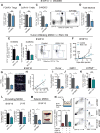
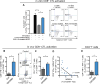

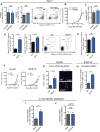
Comment in
-
LXR Agonism Depletes MDSCs to Promote Antitumor Immunity.Cancer Discov. 2018 Mar;8(3):263. doi: 10.1158/2159-8290.CD-RW2018-010. Epub 2018 Jan 19. Cancer Discov. 2018. PMID: 29352050
-
Immunotherapy: Targeting MDSCs with LXR agonists.Nat Rev Clin Oncol. 2018 Apr;15(4):200-201. doi: 10.1038/nrclinonc.2018.17. Epub 2018 Jan 31. Nat Rev Clin Oncol. 2018. PMID: 29384146 No abstract available.
References
-
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

