Recapitulation of Extracellular LAMININ Environment Maintains Stemness of Satellite Cells In Vitro
- PMID: 29337118
- PMCID: PMC5830886
- DOI: 10.1016/j.stemcr.2017.12.013
Recapitulation of Extracellular LAMININ Environment Maintains Stemness of Satellite Cells In Vitro
Abstract
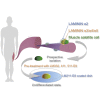
Satellite cells function as precursor cells in mature skeletal muscle homeostasis and regeneration. In healthy tissue, these cells are maintained in a state of quiescence by a microenvironment formed by myofibers and basement membrane in which LAMININs (LMs) form a major component. In the present study, we evaluated the satellite cell microenvironment in vivo and found that these cells are encapsulated by LMα2-5. We sought to recapitulate this satellite cell niche in vitro by culturing satellite cells in the presence of recombinant LM-E8 fragments. We show that treatment with LM-E8 promotes proliferation of satellite cells in an undifferentiated state, through reduced phosphorylation of JNK and p38. On transplantation into injured muscle tissue, satellite cells cultured with LM-E8 promoted the regeneration of skeletal muscle. These findings represent an efficient method of culturing satellite cells for use in transplantation through the recapitulation of the satellite cell niche using recombinant LM-E8 fragments.
Keywords: LM-E8; Laminin; cell transplantation therapy; muscle satellite cell; muscle stem cell; regeneration.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Muscle Satellite Cell Protein Teneurin-4 Regulates Differentiation During Muscle Regeneration.Stem Cells. 2015 Oct;33(10):3017-27. doi: 10.1002/stem.2058. Epub 2015 Jun 28. Stem Cells. 2015. PMID: 26013034 Free PMC article.
-
Chronic inflammation in skeletal muscle impairs satellite cells function during regeneration: can physical exercise restore the satellite cell niche?FEBS J. 2018 Jun;285(11):1973-1984. doi: 10.1111/febs.14417. Epub 2018 Mar 8. FEBS J. 2018. PMID: 29473995 Review.
-
Dormancy and quiescence of skeletal muscle stem cells.Results Probl Cell Differ. 2015;56:215-35. doi: 10.1007/978-3-662-44608-9_10. Results Probl Cell Differ. 2015. PMID: 25344673 Review.
-
Defects in glycosylation impair satellite stem cell function and niche composition in the muscles of the dystrophic Large(myd) mouse.Stem Cells. 2012 Oct;30(10):2330-41. doi: 10.1002/stem.1197. Stem Cells. 2012. PMID: 22887880 Free PMC article.
-
Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self-renewal.Nat Commun. 2018 Mar 14;9(1):1075. doi: 10.1038/s41467-018-03425-3. Nat Commun. 2018. PMID: 29540680 Free PMC article.
Cited by
-
Recent Trends in Biofabrication Technologies for Studying Skeletal Muscle Tissue-Related Diseases.Front Bioeng Biotechnol. 2021 Oct 27;9:782333. doi: 10.3389/fbioe.2021.782333. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34778240 Free PMC article. Review.
-
Generation of a MyoD knock-in reporter mouse line to study muscle stem cell dynamics and heterogeneity.iScience. 2023 Apr 8;26(5):106592. doi: 10.1016/j.isci.2023.106592. eCollection 2023 May 19. iScience. 2023. PMID: 37250337 Free PMC article.
-
Uncovering the prominent role of satellite cells in paravertebral muscle development and aging by single-nucleus RNA sequencing.Genes Dis. 2023 Feb 3;10(6):2597-2613. doi: 10.1016/j.gendis.2023.01.005. eCollection 2023 Nov. Genes Dis. 2023. PMID: 37554180 Free PMC article.
-
Isolation and Characterization of Tissue Resident CD29-Positive Progenitor Cells in Livestock to Generate a Three-Dimensional Meat Bud.Cells. 2021 Sep 21;10(9):2499. doi: 10.3390/cells10092499. Cells. 2021. PMID: 34572147 Free PMC article.
-
Attachment promoting compounds significantly enhance cell proliferation and purity of bovine satellite cells grown on microcarriers in the absence of serum.Front Bioeng Biotechnol. 2024 Nov 1;12:1443914. doi: 10.3389/fbioe.2024.1443914. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39553395 Free PMC article.
References
-
- Alter J., Rozentzweig D., Bengal E. Inhibition of myoblast differentiation by tumor necrosis factor alpha is mediated by c-Jun N-terminal kinase 1 and leukemia inhibitory factor. J. Biol. Chem. 2008;283:23224–23234. - PubMed
-
- Boonen K.J., Rosaria-Chak K.Y., Baaijens F.P., van der Schaft D.W., Post M.J. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am. J. Physiol. Cell Physiol. 2009;296:C1338–C1345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

