As Extracellular Glutamine Levels Decline, Asparagine Becomes an Essential Amino Acid
- PMID: 29337136
- PMCID: PMC5803449
- DOI: 10.1016/j.cmet.2017.12.006
As Extracellular Glutamine Levels Decline, Asparagine Becomes an Essential Amino Acid
Abstract
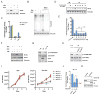
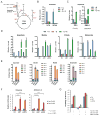
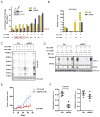
When mammalian cells are deprived of glutamine, exogenous asparagine rescues cell survival and growth. Here we report that this rescue results from use of asparagine in protein synthesis. All mammalian cell lines tested lacked cytosolic asparaginase activity and could not utilize asparagine to produce other amino acids or biosynthetic intermediates. Instead, most glutamine-deprived cell lines are capable of sufficient glutamine synthesis to maintain essential amino acid uptake and production of glutamine-dependent biosynthetic precursors, with the exception of asparagine. While experimental introduction of cytosolic asparaginase could enhance the synthesis of glutamine and increase tricarboxylic acid cycle anaplerosis and the synthesis of nucleotide precursors, cytosolic asparaginase suppressed the growth and survival of cells in glutamine-depleted medium in vitro and severely compromised the in vivo growth of tumor xenografts. These results suggest that the lack of asparaginase activity represents an evolutionary adaptation to allow mammalian cells to survive pathophysiologic variations in extracellular glutamine.
Keywords: asparaginase; asparagine; glutamine; translation.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Asparagine and Glutamine: Co-conspirators Fueling Metastasis.Cell Metab. 2018 May 1;27(5):947-949. doi: 10.1016/j.cmet.2018.04.012. Cell Metab. 2018. PMID: 29719230
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

