Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression
- PMID: 29337305
- PMCID: PMC5785251
- DOI: 10.1172/JCI96113
Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression
Erratum in
-
Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression.J Clin Invest. 2018 Apr 2;128(4):1708. doi: 10.1172/JCI120803. Epub 2018 Apr 2. J Clin Invest. 2018. PMID: 29608143 Free PMC article. No abstract available.
Abstract
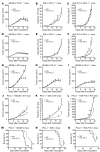
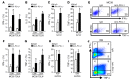
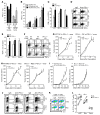
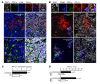
Programmed death-1 receptor (PD-L1, B7-H1) and programmed cell death protein 1 (PD-1) pathway blockade is a promising therapy for treating cancer. However, the mechanistic contribution of host and tumor PD-L1 and PD-1 signaling to the therapeutic efficacy of PD-L1 and PD-1 blockade remains elusive. Here, we evaluated 3 tumor-bearing mouse models that differ in their sensitivity to PD-L1 blockade and demonstrated a loss of therapeutic efficacy of PD-L1 blockade in immunodeficient mice and in PD-L1- and PD-1-deficient mice. In contrast, neither knockout nor overexpression of PD-L1 in tumor cells had an effect on PD-L1 blockade efficacy. Human and murine studies showed high levels of functional PD-L1 expression in dendritic cells and macrophages in the tumor microenvironments and draining lymph nodes. Additionally, expression of PD-L1 on dendritic cells and macrophages in ovarian cancer and melanoma patients correlated with the efficacy of treatment with either anti-PD-1 alone or in combination with anti-CTLA-4. Thus, PD-L1-expressing dendritic cells and macrophages may mechanistically shape and therapeutically predict clinical efficacy of PD-L1/PD-1 blockade.
Keywords: Cancer immunotherapy; Immunology.
Conflict of interest statement
Figures


Comment in
-
The host protecting the tumor from the host - targeting PD‑L1 expressed by host cells.J Clin Invest. 2018 Feb 1;128(2):570-572. doi: 10.1172/JCI99047. Epub 2018 Jan 16. J Clin Invest. 2018. PMID: 29337304 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

