OATP1B2 deficiency protects against paclitaxel-induced neurotoxicity
- PMID: 29337310
- PMCID: PMC5785270
- DOI: 10.1172/JCI96160
OATP1B2 deficiency protects against paclitaxel-induced neurotoxicity
Abstract
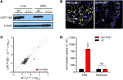
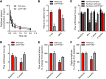
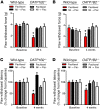
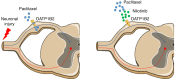
Paclitaxel is among the most widely used anticancer drugs and is known to cause a dose-limiting peripheral neurotoxicity, the initiating mechanisms of which remain unknown. Here, we identified the murine solute carrier organic anion-transporting polypeptide B2 (OATP1B2) as a mediator of paclitaxel-induced neurotoxicity. Additionally, using established tests to assess acute and chronic paclitaxel-induced neurotoxicity, we found that genetic or pharmacologic knockout of OATP1B2 protected mice from mechanically induced allodynia, thermal hyperalgesia, and changes in digital maximal action potential amplitudes. The function of this transport system was inhibited by the tyrosine kinase inhibitor nilotinib through a noncompetitive mechanism, without compromising the anticancer properties of paclitaxel. Collectively, our findings reveal a pathway that explains the fundamental basis of paclitaxel-induced neurotoxicity, with potential implications for its therapeutic management.
Keywords: Cancer; Oncology; Toxins/drugs/xenobiotics; Transport.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

