Genetic removal of eIF2α kinase PERK in mice enables hippocampal L-LTP independent of mTORC1 activity
- PMID: 29337352
- PMCID: PMC6047941
- DOI: 10.1111/jnc.14306
Genetic removal of eIF2α kinase PERK in mice enables hippocampal L-LTP independent of mTORC1 activity
Abstract
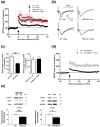
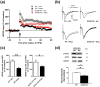
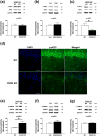
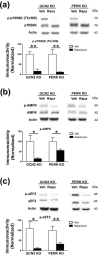
Characterization of the molecular signaling pathways underlying protein synthesis-dependent forms of synaptic plasticity, such as late long-term potentiation (L-LTP), can provide insights not only into memory expression/maintenance under physiological conditions but also potential mechanisms associated with the pathogenesis of memory disorders. Here, we report in mice that L-LTP failure induced by the mammalian (mechanistic) target of rapamycin complex 1 (mTORC1) inhibitor rapamycin is reversed by brain-specific genetic deletion of PKR-like ER kinase, PERK (PERK KO), a kinase for eukaryotic initiation factor 2α (eIF2α). In contrast, genetic removal of general control non-derepressible-2, GCN2 (GCN2 KO), another eIF2α kinase, or treatment of hippocampal slices with the PERK inhibitor GSK2606414, does not rescue rapamycin-induced L-LTP failure, suggesting mechanisms independent of eIF2α phosphorylation. Moreover, we demonstrate that phosphorylation of eukaryotic elongation factor 2 (eEF2) is significantly decreased in PERK KO mice but unaltered in GCN2 KO mice or slices treated with the PERK inhibitor. Reduction in eEF2 phosphorylation results in increased general protein synthesis, and thus could contribute to the mTORC1-independent L-LTP in PERK KO mice. We further performed experiments on mutant mice with genetic removal of eEF2K (eEF2K KO), the only known kinase for eEF2, and found that L-LTP in eEF2K KO mice is insensitive to rapamycin. These data, for the first time, connect reduction in PERK activity with the regulation of translation elongation in enabling L-LTP independent of mTORC1. Thus, our findings indicate previously unrecognized levels of complexity in the regulation of protein synthesis-dependent synaptic plasticity. Read the Editorial Highlight for this article on page 119. Cover Image for this issue: doi: 10.1111/jnc.14185.
Keywords: LTP; PERK; Rapamycin; eEF2; eIF2α; mTORC1.
© 2018 International Society for Neurochemistry.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
PERK, mTORC1 and eEF2 interplay during long term potentiation: An Editorial for 'Genetic removal of eIF2a kinase PERK in mice enables hippocampal L-LTP independent of mTORC1 activity' on page 133.J Neurochem. 2018 Jul;146(2):119-121. doi: 10.1111/jnc.14485. J Neurochem. 2018. PMID: 30133715
-
Rapamycin-Sensitive Late-LTP is Enhanced in the Hippocampus of IL-6 Transgenic Mice.Neuroscience. 2017 Dec 26;367:200-210. doi: 10.1016/j.neuroscience.2017.10.040. Epub 2017 Nov 10. Neuroscience. 2017. PMID: 29104031 Free PMC article.
-
Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2.Nature. 2005 Aug 25;436(7054):1166-73. doi: 10.1038/nature03897. Nature. 2005. PMID: 16121183 Free PMC article.
-
BDNF mechanisms in late LTP formation: A synthesis and breakdown.Neuropharmacology. 2014 Jan;76 Pt C:664-76. doi: 10.1016/j.neuropharm.2013.06.024. Epub 2013 Jul 2. Neuropharmacology. 2014. PMID: 23831365 Review.
-
BDNF-induced local protein synthesis and synaptic plasticity.Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
Cited by
-
GCN2-SLC7A11 axis coordinates autophagy, cell cycle and apoptosis and regulates cell growth in retinoblastoma upon arginine deprivation.Cancer Metab. 2024 Oct 26;12(1):31. doi: 10.1186/s40170-024-00361-3. Cancer Metab. 2024. PMID: 39462426 Free PMC article.
-
Brain-specific repression of AMPKα1 alleviates pathophysiology in Alzheimer's model mice.J Clin Invest. 2020 Jul 1;130(7):3511-3527. doi: 10.1172/JCI133982. J Clin Invest. 2020. PMID: 32213711 Free PMC article.
-
Genetic reduction of eEF2 kinase alleviates pathophysiology in Alzheimer's disease model mice.J Clin Invest. 2019 Feb 1;129(2):820-833. doi: 10.1172/JCI122954. Epub 2019 Jan 22. J Clin Invest. 2019. PMID: 30667373 Free PMC article.
-
ER Proteostasis Control of Neuronal Physiology and Synaptic Function.Trends Neurosci. 2018 Sep;41(9):610-624. doi: 10.1016/j.tins.2018.05.009. Epub 2018 Jun 23. Trends Neurosci. 2018. PMID: 29945734 Free PMC article. Review.
-
PKR: A Kinase to Remember.Front Mol Neurosci. 2019 Jan 9;11:480. doi: 10.3389/fnmol.2018.00480. eCollection 2018. Front Mol Neurosci. 2019. PMID: 30686999 Free PMC article. Review.
References
-
- Blitzer RD, Iyengar R, Landau EM. Postsynaptic signaling networks: cellular cogwheels underlying long-term plasticity. Biol Psychiatry. 2005;57:113–119. - PubMed
-
- Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. - PubMed
-
- Costa-Mattioli M, Gobert D, Harding H, Herdy B, Azzi M, Bruno M, Bidinosti M, Ben Mamou C, Marcinkiewicz E, Yoshida M, Imataka H, Cuello AC, Seidah N, Sossin W, Lacaille JC, Ron D, Nader K, Sonenberg N. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436:1166–1173. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

