Bright Side of Lignin Depolymerization: Toward New Platform Chemicals
- PMID: 29337543
- PMCID: PMC5785760
- DOI: 10.1021/acs.chemrev.7b00588
Bright Side of Lignin Depolymerization: Toward New Platform Chemicals
Abstract
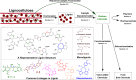
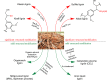
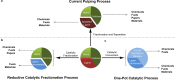
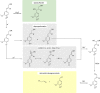
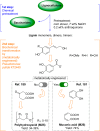
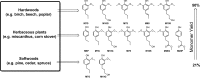
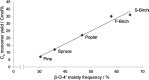
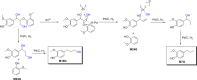
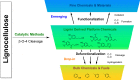
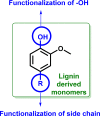
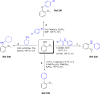
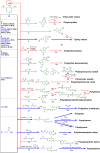
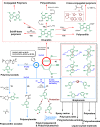
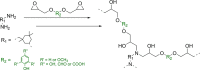
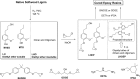
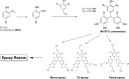
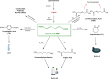
Lignin, a major component of lignocellulose, is the largest source of aromatic building blocks on the planet and harbors great potential to serve as starting material for the production of biobased products. Despite the initial challenges associated with the robust and irregular structure of lignin, the valorization of this intriguing aromatic biopolymer has come a long way: recently, many creative strategies emerged that deliver defined products via catalytic or biocatalytic depolymerization in good yields. The purpose of this review is to provide insight into these novel approaches and the potential application of such emerging new structures for the synthesis of biobased polymers or pharmacologically active molecules. Existing strategies for functionalization or defunctionalization of lignin-based compounds are also summarized. Following the whole value chain from raw lignocellulose through depolymerization to application whenever possible, specific lignin-based compounds emerge that could be in the future considered as potential lignin-derived platform chemicals.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




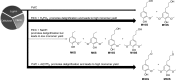
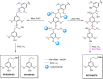
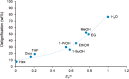
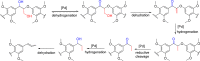


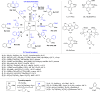
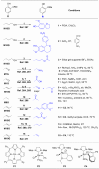
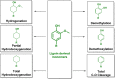
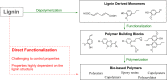



References
-
- Tye Y. Y.; Lee K. T.; Wan Abdullah W. N.; Leh C. P. The World Availability of Non-Wood Lignocellulosic Biomass for the Production of Cellulosic Ethanol and Potential Pretreatments for the Enhancement of Enzymatic Saccharification. Renewable Sustainable Energy Rev. 2016, 60, 155–172. 10.1016/j.rser.2016.01.072. - DOI
-
- Luque R.; Herrero-Davila L.; Campelo J. M.; Clark J. H.; Hidalgo J. M.; Luna D.; Marinas J. M.; Romero A. A. Biofuels: A Technological Perspective. Energy Environ. Sci. 2008, 1, 542–564. 10.1039/b807094f. - DOI
-
- Dapsens P. Y.; Mondelli C.; Pérez-Ramírez J. Biobased Chemicals from Conception toward Industrial Reality: Lessons Learned and to Be Learned. ACS Catal. 2012, 2, 1487–1499. 10.1021/cs300124m. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

