Specific Molecular Signatures for Type II Crustins in Penaeid Shrimp Uncovered by the Identification of Crustin-Like Antimicrobial Peptides in Litopenaeus vannamei
- PMID: 29337853
- PMCID: PMC5793079
- DOI: 10.3390/md16010031
Specific Molecular Signatures for Type II Crustins in Penaeid Shrimp Uncovered by the Identification of Crustin-Like Antimicrobial Peptides in Litopenaeus vannamei
Abstract
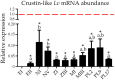
Crustins form a large family of antimicrobial peptides (AMPs) in crustaceans composed of four sub-groups (Types I-IV). Type II crustins (Type IIa or "Crustins" and Type IIb or "Crustin-like") possess a typical hydrophobic N-terminal region and are by far the most representative sub-group found in penaeid shrimp. To gain insight into the molecular diversity of Type II crustins in penaeids, we identified and characterized a Type IIb crustin in Litopenaeus vannamei (Crustin-like Lv) and compared Type II crustins at both molecular and transcriptional levels. Although L. vannamei Type II crustins (Crustin Lv and Crustin-like Lv) are encoded by separate genes, they showed a similar tissue distribution (hemocytes and gills) and transcriptional response to the shrimp pathogens Vibrio harveyi and White spot syndrome virus (WSSV). As Crustin Lv, Crustin-like Lv transcripts were found to be present early in development, suggesting a maternal contribution to shrimp progeny. Altogether, our in silico and transcriptional data allowed to conclude that (1) each sub-type displays a specific amino acid signature at the C-terminal end holding both the cysteine-rich region and the whey acidic protein (WAP) domain, and that (2) shrimp Type II crustins evolved from a common ancestral gene that conserved a similar pattern of transcriptional regulation.
Keywords: WAP domain; crustacean; host defense peptide; host-pathogen interaction; invertebrate immunity; molecular diversity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

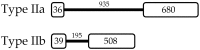




References
-
- Bartlett T.C., Cuthbertson B.J., Shepard E.F., Chapman R.W., Gross P.S., Warr G.W. Crustins, homologues of an 11.5-kDa antibacterial peptide, from two species of penaeid shrimp, Litopenaeus vannamei and Litopenaeus setiferus. Mar. Biotechnol. 2002;4:278–293. doi: 10.1007/s10126-002-0020-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

