Subcellular Reactive Oxygen Species (ROS) in Cardiovascular Pathophysiology
- PMID: 29337890
- PMCID: PMC5789324
- DOI: 10.3390/antiox7010014
Subcellular Reactive Oxygen Species (ROS) in Cardiovascular Pathophysiology
Abstract
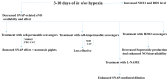
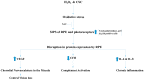
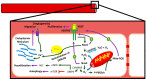
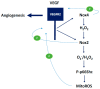
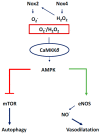
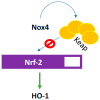
There exist two opposing perspectives regarding reactive oxygen species (ROS) and their roles in angiogenesis and cardiovascular system, one that favors harmful and causal effects of ROS, while the other supports beneficial effects. Recent studies have shown that interaction between ROS in different sub-cellular compartments plays a crucial role in determining the outcomes (beneficial vs. deleterious) of ROS exposures on the vascular system. Oxidant radicals in one cellular organelle can affect the ROS content and function in other sub-cellular compartments in endothelial cells (ECs). In this review, we will focus on a critical fact that the effects or the final phenotypic outcome of ROS exposure to EC are tissue- or organ-specific, and depend on the spatial (subcellular localization) and temporal (duration of ROS exposure) modulation of ROS levels.
Keywords: Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase; ROS; angiogenesis; cardiovascular disease; coronary endothelium; mitochondrial ROS.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
The Relationship Between Reactive Oxygen Species and Endothelial Cell Metabolism.Front Chem. 2020 Nov 26;8:592688. doi: 10.3389/fchem.2020.592688. eCollection 2020. Front Chem. 2020. PMID: 33330380 Free PMC article. Review.
-
Reactive oxygen species and endothelial function--role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases.Basic Clin Pharmacol Toxicol. 2012 Jan;110(1):87-94. doi: 10.1111/j.1742-7843.2011.00785.x. Epub 2011 Sep 28. Basic Clin Pharmacol Toxicol. 2012. PMID: 21883939 Review.
-
Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase.Mol Cell Biochem. 2004 Sep;264(1-2):85-97. doi: 10.1023/b:mcbi.0000044378.09409.b5. Mol Cell Biochem. 2004. PMID: 15544038 Review.
-
Pharmacological strategies to lower crosstalk between nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and mitochondria.Biomed Pharmacother. 2019 Mar;111:1478-1498. doi: 10.1016/j.biopha.2018.11.128. Epub 2019 Feb 14. Biomed Pharmacother. 2019. PMID: 30841463 Review.
-
Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase.Circ Res. 2004 Oct 15;95(8):773-9. doi: 10.1161/01.RES.0000145728.22878.45. Epub 2004 Sep 23. Circ Res. 2004. PMID: 15388638
Cited by
-
The relationship of redox signaling with the risk for atherosclerosis.Front Pharmacol. 2024 Aug 1;15:1430293. doi: 10.3389/fphar.2024.1430293. eCollection 2024. Front Pharmacol. 2024. PMID: 39148537 Free PMC article. Review.
-
Peripheral Blood Mononuclear Cells Antioxidant Adaptations to Regular Physical Activity in Elderly People.Nutrients. 2018 Oct 20;10(10):1555. doi: 10.3390/nu10101555. Nutrients. 2018. PMID: 30347790 Free PMC article.
-
Ceramide and Regulation of Vascular Tone.Int J Mol Sci. 2019 Jan 18;20(2):411. doi: 10.3390/ijms20020411. Int J Mol Sci. 2019. PMID: 30669371 Free PMC article. Review.
-
Pleiotropic and Potentially Beneficial Effects of Reactive Oxygen Species on the Intracellular Signaling Pathways in Endothelial Cells.Antioxidants (Basel). 2021 Jun 3;10(6):904. doi: 10.3390/antiox10060904. Antioxidants (Basel). 2021. PMID: 34205032 Free PMC article. Review.
-
Say NO to ROS: Their Roles in Embryonic Heart Development and Pathogenesis of Congenital Heart Defects in Maternal Diabetes.Antioxidants (Basel). 2019 Oct 1;8(10):436. doi: 10.3390/antiox8100436. Antioxidants (Basel). 2019. PMID: 31581464 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

