Targeting HER2 in colorectal cancer: The landscape of amplification and short variant mutations in ERBB2 and ERBB3
- PMID: 29338072
- PMCID: PMC5900732
- DOI: 10.1002/cncr.31125
Targeting HER2 in colorectal cancer: The landscape of amplification and short variant mutations in ERBB2 and ERBB3
Abstract
Background: In contrast to lung cancer, few precision treatments are available for colorectal cancer (CRC). One rapidly emerging treatment target in CRC is ERBB2 (human epidermal growth factor receptor 2 [HER2]). Oncogenic alterations in HER2, or its dimerization partner HER3, can underlie sensitivity to HER2-targeted therapies.
Methods: In this study, 8887 CRC cases were evaluated by comprehensive genomic profiling for genomic alterations in 315 cancer-related genes, tumor mutational burden, and microsatellite instability. This cohort included both colonic (7599 cases; 85.5%) and rectal (1288 cases; 14.5%) adenocarcinomas.
Results: A total of 569 mCRCs were positive for ERBB2 (429 cases; 4.8%) and/or ERBB3 (148 cases; 1.7%) and featured ERBB amplification, short variant alterations, or a combination of the 2. High tumor mutational burden (≥20 mutations/Mb) was significantly more common in ERBB-mutated samples, and ERBB3-mutated CRCs were significantly more likely to have high microsatellite instability (P<.002). Alterations affecting KRAS (27.3%) were significantly underrepresented in ERBB2-amplified samples compared with wild-type CRC samples (51.8%), and ERBB2- or ERBB3-mutated samples (49.0% and 60.8%, respectively) (P<.01). Other significant differences in mutation frequency were observed for genes in the PI3K/MTOR and mismatch repair pathways.
Conclusions: Although observed less often than in breast or upper gastrointestinal carcinomas, indications for which anti-HER2 therapies are approved, the percentage of CRC with ERBB genomic alterations is significant. Importantly, 32% of ERBB2-positive CRCs harbor short variant alterations that are undetectable by routine immunohistochemistry or fluorescence in situ hybridization testing. The success of anti-HER2 therapies in ongoing clinical trials is a promising development for patients with CRC. Cancer 2018;124:1358-73. © 2018 Foundation Medicine, Inc. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Keywords: ERBB2, ERBB3; colorectal adenocarcinoma; comprehensive genomic profiling; human epidermal growth factor receptor 2 (HER2); lapatinib; microsatellite instability; pertuzumab; trastuzumab; tumor mutational burden.
© 2018 Foundation Medicine, Inc. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Figures

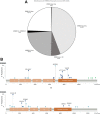


References
-
- Schechter AL, Stern DF, Vaidyanathan L, et al. The neu oncogene: an erb‐B‐related gene encoding a 185,000‐Mr tumour antigen. Nature. 1984;312:513‐516. - PubMed
-
- Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER‐2 receptor and breast cancer: ten years of targeted anti‐HER‐2 therapy and personalized medicine. Oncologist. 2009;14:320‐368. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al; ToGA Trial Investigators . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet. 2010;376:687‐697. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

