Human Umbilical Cord Mesenchymal Stem Cells Preserve Adult Newborn Neurons and Reduce Neurological Injury after Cerebral Ischemia by Reducing the Number of Hypertrophic Microglia/Macrophages
- PMID: 29338384
- PMCID: PMC5784525
- DOI: 10.1177/0963689717728936
Human Umbilical Cord Mesenchymal Stem Cells Preserve Adult Newborn Neurons and Reduce Neurological Injury after Cerebral Ischemia by Reducing the Number of Hypertrophic Microglia/Macrophages
Abstract
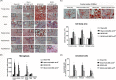
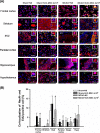
Microglia are the first source of a neuroinflammatory cascade, which seems to be involved in every phase of stroke-related neuronal damage. Two weeks after transient middle cerebral artery occlusion (MCAO), vehicle-treated rats displayed higher numbers of total ionized calcium-binding adaptor molecule 1 (Iba-1)-positive cells, greater cell body areas of Iba-1-positive cells, and higher numbers of hypertrophic Iba-1-positive cells (with a cell body area over 80 μm2) in the ipsilateral ischemic brain regions including the frontal cortex, striatum, and parietal cortex. In addition, MCAO decreased the number of migrating neuroblasts (or DCX- and 5-ethynyl-2'-deoxyuridine-positive cells) in the cortex, subventricular zone, and hippocampus of the ischemic brain, followed by neurological injury (including brain infarct and neurological deficits). Intravenous administration of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs; 1 × 106 or 4 × 106) at 24 h after MCAO reduced neurological injury, decreased the number of hypertrophic microglia/macrophages, and increased the number of newborn neurons in rat brains. Thus, the accumulation of hypertrophic microglia/macrophages seems to be detrimental to neurogenesis after stroke. Treatment with hUC-MSCs preserved adult newborn neurons and reduced functional impairment after transient cerebral ischemia by reducing the number of hypertrophic microglia/macrophages.
Keywords: cell therapy; microglia; stroke; umbilical cord mesenchymal stem cells.
Conflict of interest statement
Figures




References
-
- Whiteley WN, Slot KB, Fernandes P, Sandercock P, Wardlaw J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke. 2012;43(11):2904–2909. - PubMed
-
- McGuckin CP, Jurga M, Miller AM, Sarnowska A, Wiedner M, Boyle NT, Lynch MA, Jablonska A, Drela K, Lukomska B, et al. Ischemic brain injury: a consortium analysis of key factors involved in mesenchymal stem cell-mediated inflammatory reduction. Arch Biochem Biophys. 2013;534(1-2):88–97. - PubMed
-
- Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23–44. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

