Macrophage polarization and activation at the interface of multi-walled carbon nanotube-induced pulmonary inflammation and fibrosis
- PMID: 29338488
- PMCID: PMC6413511
- DOI: 10.1080/17435390.2018.1425501
Macrophage polarization and activation at the interface of multi-walled carbon nanotube-induced pulmonary inflammation and fibrosis
Abstract
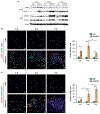
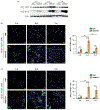
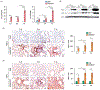
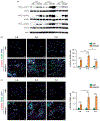
Pulmonary exposure to carbon nanotubes (CNTs) induces fibrosing lesions in the lungs that manifest rapid-onset inflammatory and fibrotic responses, leading to chronic fibrosis in animals and health concerns in exposed humans. The mechanisms underlying CNT-induced fibrogenic effects remain undefined. Macrophages are known to play important roles in immune regulation and fibrosis development through their distinct subsets. Here we investigated macrophage polarization and activation in mouse lungs exposed to multi-walled CNTs (MWCNTs). Male C57BL/6J mice were treated with MWCNTs (XNRI MWNT-7) at 40 μg per mouse (∼1.86 mg/kg body weight) by oropharyngeal aspiration. The treatment stimulated prominent acute inflammatory and fibrotic responses. Moreover, it induced pronounced enrichment and polarization of macrophages with significantly increased M1 and M2 populations in a time-dependent manner. Induction of M1 polarization was apparent on day 1 with a peak on day 3, but declined rapidly thereafter. On the other hand, the M2 polarization was induced on day 1 modestly, but was remarkably elevated on day 3 and maintained at a high level through day 7. M1 and M2 macrophages were functionally activated by MWCNTs as indicated by the expression of their distinctive functional markers, such as iNOS and ARG1, with time courses parallel to M1 and M2 polarization, respectively. Molecular analysis revealed MWCNTs boosted specific STAT and IRF signaling pathways to regulate M1 and M2 polarization in the lungs. These findings suggest a new mechanistic connection between inflammation and fibrosis induced by MWCNTs through the polarization and activation of macrophages during MWCNT-induced lung pathologic response.
Keywords: M1–M2 polarization; fibrosis; inflammation; macrophage; multi-walled carbon nanotube.
Conflict of interest statement
Disclosure statement
The authors declare there are no competing financial interests.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Figures


Similar articles
-
Resolution of Pulmonary Inflammation Induced by Carbon Nanotubes and Fullerenes in Mice: Role of Macrophage Polarization.Front Immunol. 2020 Jun 12;11:1186. doi: 10.3389/fimmu.2020.01186. eCollection 2020. Front Immunol. 2020. PMID: 32595644 Free PMC article.
-
In vivo activation of a T helper 2-driven innate immune response in lung fibrosis induced by multi-walled carbon nanotubes.Arch Toxicol. 2016 Sep;90(9):2231-2248. doi: 10.1007/s00204-016-1711-1. Epub 2016 Apr 22. Arch Toxicol. 2016. PMID: 27106021 Free PMC article.
-
Osteopontin enhances multi-walled carbon nanotube-triggered lung fibrosis by promoting TGF-β1 activation and myofibroblast differentiation.Part Fibre Toxicol. 2017 Jun 8;14(1):18. doi: 10.1186/s12989-017-0198-0. Part Fibre Toxicol. 2017. PMID: 28595626 Free PMC article.
-
Type 2 Immune Mechanisms in Carbon Nanotube-Induced Lung Fibrosis.Front Immunol. 2018 May 22;9:1120. doi: 10.3389/fimmu.2018.01120. eCollection 2018. Front Immunol. 2018. PMID: 29872441 Free PMC article. Review.
-
Signaling Pathways Implicated in Carbon Nanotube-Induced Lung Inflammation.Front Immunol. 2020 Dec 11;11:552613. doi: 10.3389/fimmu.2020.552613. eCollection 2020. Front Immunol. 2020. PMID: 33391253 Free PMC article. Review.
Cited by
-
Sex differences in the inflammatory immune response to multi-walled carbon nanotubes and crystalline silica.Inhal Toxicol. 2019 Jun;31(7):285-297. doi: 10.1080/08958378.2019.1669743. Epub 2019 Sep 26. Inhal Toxicol. 2019. PMID: 31556754 Free PMC article.
-
Microenvironmental Alterations in Carbon Nanotube-Induced Lung Inflammation and Fibrosis.Front Cell Dev Biol. 2020 Feb 28;8:126. doi: 10.3389/fcell.2020.00126. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32185174 Free PMC article. Review.
-
Centrality of Myeloid-Lineage Phagocytes in Particle-Triggered Inflammation and Autoimmunity.Front Toxicol. 2021 Nov 4;3:777768. doi: 10.3389/ftox.2021.777768. eCollection 2021. Front Toxicol. 2021. PMID: 35295146 Free PMC article. Review.
-
Comparative in Vitro Cytotoxicity of Realistic Doses of Benchmark Multi-Walled Carbon Nanotubes towards Macrophages and Airway Epithelial Cells.Nanomaterials (Basel). 2019 Jul 6;9(7):982. doi: 10.3390/nano9070982. Nanomaterials (Basel). 2019. PMID: 31284615 Free PMC article.
-
Role of A2B adenosine receptor-dependent adenosine signaling in multi-walled carbon nanotube-triggered lung fibrosis in mice.J Nanobiotechnology. 2019 Mar 29;17(1):45. doi: 10.1186/s12951-019-0478-y. J Nanobiotechnology. 2019. PMID: 30922349 Free PMC article.
References
-
- Aiso S, Yamazaki K, Umeda Y, Asakura M, Kasai T, Takaya M, Toya T, et al. 2010. “Pulmonary Toxicity of Intratracheally Instilled Multiwall Carbon Nanotubes in Male Fischer 344 Rats.” Industrial Health 48:783–795. http://www.ncbi.nlm.nih.gov/pubmed/20616469 - PubMed
-
- Beamer CA, Girtsman TA, Seaver BP, Finsaas KJ, Migliaccio CT, Perry VK, Rottman JB, et al. 2013. “IL-33 Mediates Multi-walled Carbon Nanotube (MWCNT)-induced Airway Hyper-reactivity Via the Mobilization of Innate Helper Cells in the Lung.” Nanotoxicology 7:1070–1081. doi:10.3109/17435390.2012.702230 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
