Predictors of remission with etanercept-methotrexate induction therapy and loss of remission with etanercept maintenance, reduction, or withdrawal in moderately active rheumatoid arthritis: results of the PRESERVE trial
- PMID: 29338762
- PMCID: PMC5771183
- DOI: 10.1186/s13075-017-1484-9
Predictors of remission with etanercept-methotrexate induction therapy and loss of remission with etanercept maintenance, reduction, or withdrawal in moderately active rheumatoid arthritis: results of the PRESERVE trial
Abstract
Background: The aim was to analyze characteristics that predict remission induction and subsequent loss of remission in patients with moderately active rheumatoid arthritis (RA) who received full-dose combination etanercept plus methotrexate induction therapy followed by reduced-dose etanercept or etanercept withdrawal.
Methods: Patients with Disease Activity Score based on 28-joint count (DAS28) >3.2 and ≤5.1 received open-label etanercept 50 mg once weekly (QW) plus methotrexate for 36 weeks. Those who achieved DAS28 low disease activity by 36 weeks were randomized to double-blind treatment with etanercept 50 mg or 25 mg QW plus methotrexate or placebo plus methotrexate for 52 weeks. All analyses were adjusted for the continuous baseline variables of their respective remission outcomes.
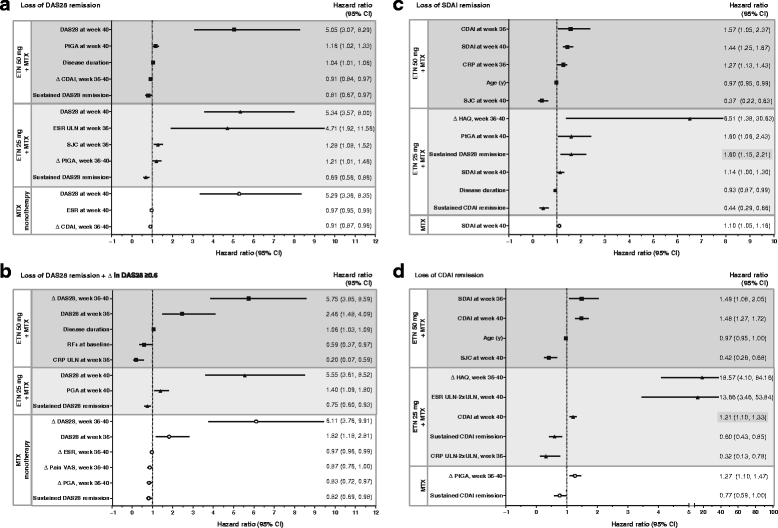
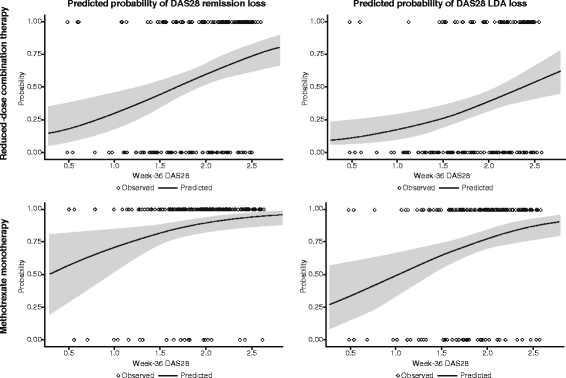
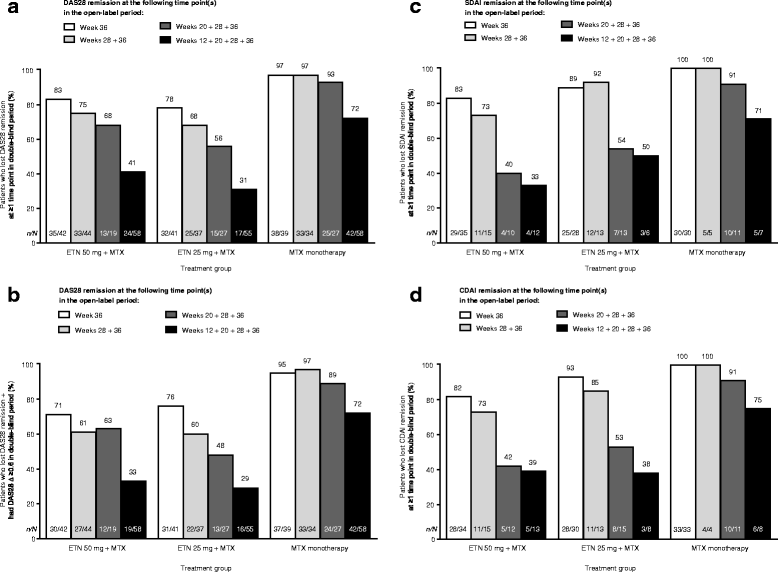
Results: Younger age, body mass index (BMI) <30 kg/m2, and lower Health Assessment Questionnaire (HAQ) score at baseline were significant predictors of week-36 remission (P < 0.05) based on DAS28, Simplified Disease Activity Index (SDAI), and Clinical Disease Activity Index (CDAI). Baseline DAS28, SDAI, and CDAI were significantly predictive of all three remission endpoints (P < 0.05). For all three treatments, the strongest predictors of loss of DAS28 remission included failure to achieve sustained remission (DAS28 < 2.6 at weeks 12, 20, 28, and 36) with induction therapy, higher DAS28/SDAI/CDAI at randomization and at 1 month, increase in DAS28/SDAI/CDAI at 1 month, and increase in DAS28/CDAI/SDAI components and patient-reported outcomes (PROs) at 1 month. With the exception of not achieving sustained remission, very similar significant predictors were observed for loss of SDAI and CDAI remission.
Conclusion: These findings suggest that patients with moderately active RA who are younger and have lower BMI, lower HAQ, and lower disease activity at baseline are most likely to achieve remission when receiving combination etanercept and methotrexate induction therapy. In addition, patients who fail to achieve sustained remission with induction therapy and those with worse disease activity and PROs at early time points after initiating maintenance therapy with a full-dose or reduced-dose etanercept-methotrexate regimen or methotrexate monotherapy are most likely to lose remission across all treatment arms. These findings may help guide clinicians' decision-making as they treat patients to remission and beyond.
Trial registration: ClinicalTrials.gov, NCT00565409 . Registered on 28 November 2007.
Keywords: Etanercept; Low disease activity; Methotrexate; Remission; Rheumatoid arthritis; Treatment.
Conflict of interest statement
Ethics approval and consent to participate
The PRESERVE trial was conducted in accordance with the ethical principles of the Declaration of Helsinki and the guidelines for Good Clinical Practice of the International Conference on Harmonisation. All patients were required to sign an informed consent form. The institutional review board or independent ethics committee at the following participating centers reviewed and approved all consent forms and the PRESERVE study protocol: Australia – Bellberry Limited, Dulwich, SA; Flinders Clinical Research Ethics Committee, Repatriation General Hospital, Daw Park, SA; The Austin Health Human Research Ethics Committee, Heidelberg, VIC. Austria – Ethikkommission der Stadt Wien, Wien. Belgium – Comite d'ethique Hospitalo-Facultaire Universitaire de Liege, Centre Hospitalier Universitaire du Sart Tilman, Liege; CHU Liege, Comité d’Ethique de la Faculté de Médicine, Centre Hospitalier Universitaire du Sart Tilman, Liege. Chile – Comite Etico Cientifico, Servicio de Salud Metropolitano Oriente, Santiago, RM. Colombia – Comite de etica de la fundacion instituto de reumatologia Fernando Chalem, Bogota, Cundinamarca; Comité de etica independiente centro de reumatologia y ortopedia, Barranquilla, Atlantico; Comite de Etica de la Investigacion Riesgo de Fractura S.A., Bogota, Cundinamarca. Czech Republic – Eticka komise Fakultni nemocnice Olomouc a Lekarske fakulty, Univerzity Palackeho, Olomouc; Ethics Committee of Institute of Rheumatology, Praha; Eticka komise pro multicentricka klinicka hodnoceni Fakultni nemocnice v Motole, Praha; Multicentricka eticka komise Fakultni nemocnice u sv. Anny v Brne - detasovane pracoviste, Brno. France – Comité de Protection des Personnes, Sud-Ouest et Outre-Mer II Hôpital Purpan, Toulouse Cedex 9. Germany – Ethik-Kommission bei der Medizinischen Fakultaet der Universitaet Wuerzburg, Institut fuer Pharmakologie und Toxikologie, Wuerzburg. Hungary – Egeszsegugyi Tudomanyos Tanacs Klinikai Farmakologiai Etikai Bizottsaga, Budapest. Italy – Comitato Etico Scientifico dell'Azienda Ospedaliera Ospedale Niguarda Ca' Granda di Milano, Milano; Comitato Etico Azienda Ospedaliera Universitaria San Luigi Gonzaga di Orbassano, Orbassano; Comitato Etico dell'Universita' Campus Bio-Medico di Roma, Roma; Comitato Etico dell'Azienda Ospedali Vittorio Emanuele, Ferrarotto, S.Bambino, Catania. Republic of Korea – Institutional Review Board, Gangnam Severance Hospital, Seoul; Institutional Review Board/Ethics Commitee of Kangdong Sacred Heart Hospital, Seoul; Hallym University Sacred Heart Hospital Institutional Review Board, Anyang-Si, Gyeonggi-do; Institutional Review Board for Human Research, Konkuk University Hospital, Seoul; Institutional Review Board, Gachon Gil Medical Center, Incheon; Hanyang University Hospital IRB, Seoul; IRB of Eulji University Hospital, Daejeon. Mexico – Comite de Etica de la Facultad de Medidna de la UANL Hospital Universitario Dr Jose Eleuterio Gonzalez, Monterrey NL; Comite Bioetico para la Investigacion Clinica S. C., Puebla 422, Mexico, DF; Comite de Etica del Hospital San Javier, Guadalajara, Jalisco; Comité de Investigación del Hospital CEM, Merida, Yucatán. The Netherlands – Independent Review Board Nijmegen (IRBN) Nijmegen, Wijchen. Poland – Komisja Etyczna Przy Instytucie Reumatologicznym ul Spartanska 1, Warszawa. Russian Federation - Biomedical Ethics Committee at FGU "Severo-Zapadny District Medical Center of Rozdrav," St. Petersburg; Ethical council under responsibility of department of state regulation of circulation of medicines, Ministry of Public Health and Social Development of Russian Federation, Moscow; Local Ethics Committee at GU Institute of Rheumatology of RAMS, Moscow; Local Ethics Committee at GUZ "City Clinical Hospital #1 n.a., N.I.Pirogov," Rheumatology Department, Moscow; Local Ethics Committee at GUZ at Leningrad Regional Clinical Hospital, St. Petersburg; Local Ethics Committee at Moscow Regional Research Clinical Institute, n.a. M.F. Vladimirskogo, Moscow. Serbia and Montenegro – Ethics Committee of Institute of Rheumatology-Belgrade, Belgrade; Ethics Committee of Institute for Treatment and Rehabilitation "Niska Banja," Niska Banja; Ethics Committee Military Medical Academy, Belgrade. Spain – Hospital General Universitario Gregorio Marañon, Comite etico de Investigacion Clinica c/Doctor, Madrid. Sweden – Regionala etikprovningsnamnden i Uppsala, Uppsala. Taiwan – Institutional Review Board of Tri-Service General Hospital National Defense Medical Center, Neihu, Taipei; Kaohsiung Medical University Chung-Ho Memorial Hospital, Institutional Review Board, Kaohsiung. United Kingdom – Liverpool Adult Research Ethics Committee, Research Ethics Office, Liverpool; Wrightington, Wigan and Leigh LREC, Greater Manchester Strategic Health Authority, Manchester; West Midlands Research Ethics Committee, Redditch, Worcestershire.
Consent for publication
Not applicable.
Competing interests
JSS has received research grants and consulting fees from AbbVie, BMS, MSD, Pfizer, and Roche, and consulting fees from Astra-Zeneca, Boehringer Ingelheim, Celgene, Celtrion, GSK, ILTOO, Janssen, Lilly, Novartis-Sandoz, Samsung, and UCB. AS is an employee of inVentiv Health and was contracted by Pfizer to provide statistical support for the development of this paper. ASK, TVJ, and LM are employees of Pfizer and hold Pfizer stock. The authors do not report any non-financial conflicts of interest.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Smolen JS, Han C, van der Heijde DM, Emery P, Bathon JM, Keystone E, Maini RN, Kalden JR, Aletaha D, Baker D, et al. Radiographic changes in rheumatoid arthritis patients attaining different disease activity states with methotrexate monotherapy and infliximab plus methotrexate: the impacts of remission and tumour necrosis factor blockade. Ann Rheum Dis. 2009;68(6):823–7. doi: 10.1136/ard.2008.090019. - DOI - PubMed
-
- van der Heijde D, Landewé R, van Vollenhoven R, Fatenejad S, Klareskog L. Level of radiographic damage and radiographic progression are determinants of physical function: a longitudinal analysis of the TEMPO trial. Ann Rheum Dis. 2008;67(9):1267–70. doi: 10.1136/ard.2007.081331. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

