Evaluation of two health education interventions to improve the varicella vaccination: a randomized controlled trial from a province in the east China
- PMID: 29338782
- PMCID: PMC5771153
- DOI: 10.1186/s12889-018-5070-0
Evaluation of two health education interventions to improve the varicella vaccination: a randomized controlled trial from a province in the east China
Abstract
Background: We evaluated the effect of two Elaboration Likelihood Model (ELM)-based health educational interventions on varicella vaccine (VarV) vaccination among pregnant women in a province in the east China.
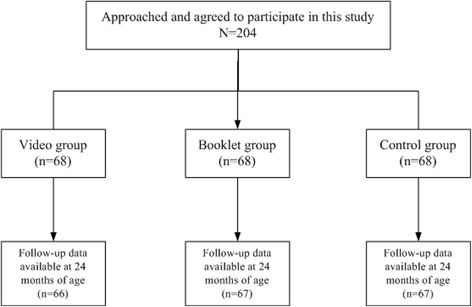
Methods: A prospective randomized controlled trial was conducted among 200 pregnant women with ≥12 gestation weeks to test two interventions, including a messaging video and a messaging booklet. The participants were randomly assigned into the control group, the video group or the booklet group. The VarV coverage at 12 and 24 months old was compared among the children of the three groups and relative risks (RRs) were calculated, by using the coverage of the control group as reference. The timeliness of VarV was also assessed. Furthermore, differences in the effects on the knowledge and attitude of VarV vaccination between the two interventions was evaluated.
Results: The VarV coverage of their children by 24 months of age was 86.4%, 76.1% and 56.7% for the video group, the booklet group and the control group, respectively. The relative risks (RRs) for the coverage of VarV at 24 months of age were 4.8 (95% CI: 2.06-11.3) for the video group and 2.4 (95% CI: 1.2-5.1) for the booklet group. The means of delays were 57.3 days in the video group, 76.9 days in the booklet group, and 100.6 days in the control group. The proportion of women who intended to vaccinate their children with VarV was higher in the video group than the booklet group (93.9% vs. 82.1%, p < 0.05).
Conclusions: Our findings indicated that perinatal health education through booklet or video could improve the coverage and schedule adherence for children's VarV vaccination.
Keywords: Effect; Evaluation; Health education; Randomized controlled trial; Varicella vaccine.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the ethical review board of Zhejiang provincial center for disease control and prevention. Written informed consent was obtained from every pregnant woman once there was a decision to participate.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Li Q, Hu Y, Zhong Y, Chen Y, Tang X, Guo J, Shen L. Using the immunization information system to determine vaccination coverage rates among children aged 1-7 years: a report from Zhejiang Province, China. Int J Environ Res Public Health. 2014;11(3):2713–2728. doi: 10.3390/ijerph110302713. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical