High-throughput immunophenotypic characterization of bone marrow- and cord blood-derived mesenchymal stromal cells reveals common and differentially expressed markers: identification of angiotensin-converting enzyme (CD143) as a marker differentially expressed between adult and perinatal tissue sources
- PMID: 29338788
- PMCID: PMC5771027
- DOI: 10.1186/s13287-017-0755-3
High-throughput immunophenotypic characterization of bone marrow- and cord blood-derived mesenchymal stromal cells reveals common and differentially expressed markers: identification of angiotensin-converting enzyme (CD143) as a marker differentially expressed between adult and perinatal tissue sources
Abstract
Background: Mesenchymal stromal cells (MSC) are a heterogeneous population of multipotent progenitors used in the clinic because of their immunomodulatory properties and their ability to differentiate into multiple mesodermal lineages. Although bone marrow (BM) remains the most common MSC source, cord blood (CB) can be collected noninvasively and without major ethical concerns. Comparative studies comprehensively characterizing the MSC phenotype across several tissue sources are still lacking. This study provides a 246-antigen immunophenotypic analysis of BM- and CB-derived MSC aimed at identifying common and strongly expressed MSC markers as well as the existence of discriminating markers between the two sources.
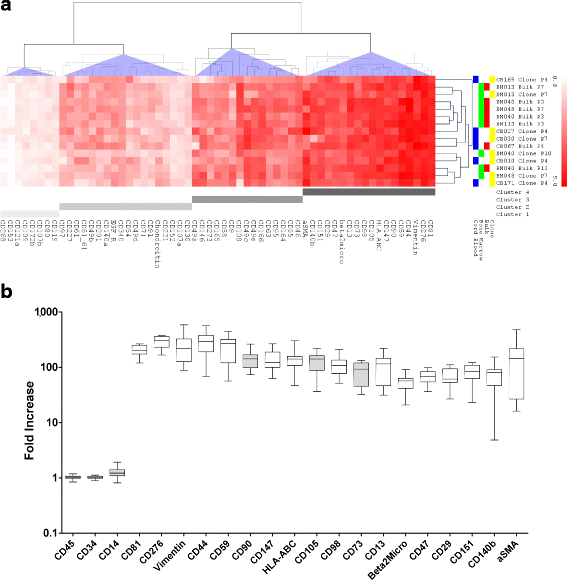
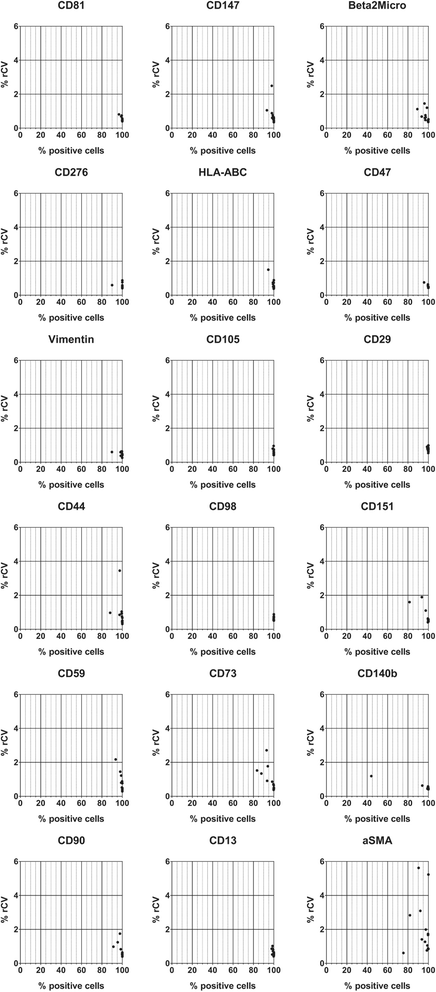
Methods: BM-MSC (n = 4) were expanded and analyzed as bulk (n = 6) or single clones isolated from the bulk culture (n = 3). CB-MSC (n = 6) were isolated and expanded as single clones in 5/6 samples. The BM-MSC and CB-MSC phenotype was investigated by flow cytometry using a panel of 246 monoclonal antibodies. To define the markers common to both sources, those showing the smallest variation between samples (coefficient of variation of log2 fold increase ≤ 0.5, n = 59) were selected for unsupervised hierarchical cluster analysis (HCL). Differentially expressed markers were identified by directly comparing the expression of all 246 antigens between BM-MSC and CB-MSC.
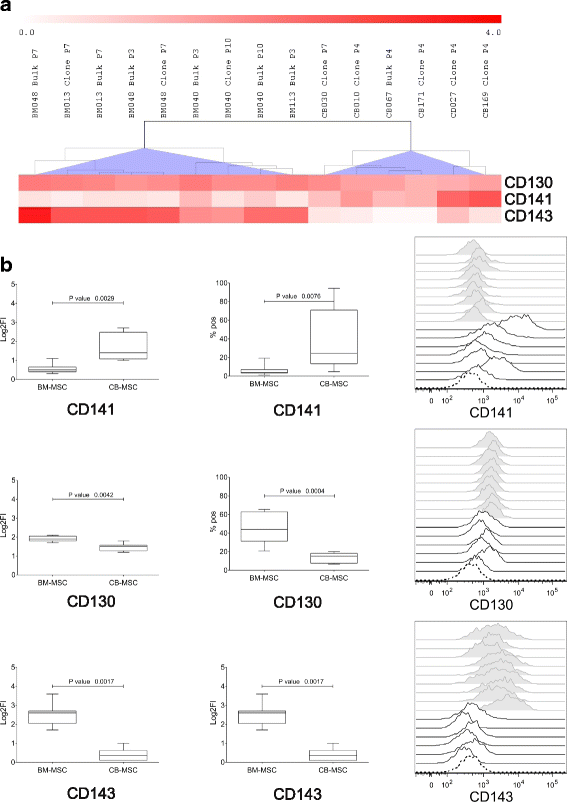
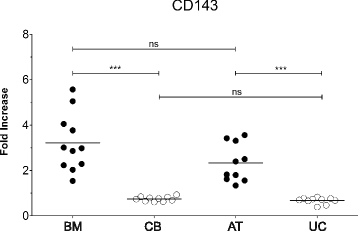
Results: Based on HCL, 18 markers clustered as strongly expressed in BM-MSC and CB-MSC, including alpha-smooth muscle antigen (SMA), beta-2-microglobulin, CD105, CD13, CD140b, CD147, CD151, CD276, CD29, CD44, CD47, CD59, CD73, CD81, CD90, CD98, HLA-ABC, and vimentin. All except CD140b and alpha-SMA were suitable for the specific identification of ex-vivo expanded MSC. Notably, only angiotensin-converting enzyme (CD143) was exclusively expressed on BM-MSC. CD143 expression was tested on 10 additional BM-MSC and CB-MSC and on 10 umbilical cord- and adipose tissue-derived MSC samples, confirming that its expression is restricted to adult sources.
Conclusions: This is the first study that has comprehensively compared the phenotype of BM-MSC and CB-MSC. We have identified markers that could complement the minimal panel proposed for the in-vitro MSC definition, being shared and strongly expressed by BM- and CB-derived MSC. We have also identified CD143 as a marker exclusively expressed on MSC derived from adult tissue sources. Further studies will elucidate the biological role of CD143 and its potential association with tissue-specific MSC features.
Keywords: Angiotensin-converting enzyme; Bone marrow; CD143; Cord blood; Flow cytometry; Hematopoietic progenitor cell marker; High-throughput screening; Immunophenotype; Lyoplate; Mesenchymal stromal cells.
Conflict of interest statement
Ethics approval and consent to participate
The present study was approved (reference protocol SIT-VR 13/73) and informed consent for cord blood collection and donation for research was received from the mothers (specific section in the form IBMDR SCO101, version 2, January 2013). All collection procedures were approved by the ethics committee of S. Bortolo Hospital, Vicenza, Italy (Act 40/09 of 16 December 2009).
Consent for publication
Informed consent included the consent for publication.
Competing interests
GR has supported the present work in his function as a Becton Dickinson Italia associate. All remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

