Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes
- PMID: 29338792
- PMCID: PMC5771074
- DOI: 10.1186/s12931-017-0710-y
Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes
Abstract
Background: Inhaled drug delivery is the cornerstone treatment for asthma and chronic obstructive pulmonary disease (COPD). However, use of inhaler devices can be challenging, potentially leading to critical errors in handling that can significantly reduce drug delivery to the lungs and effectiveness of treatment.
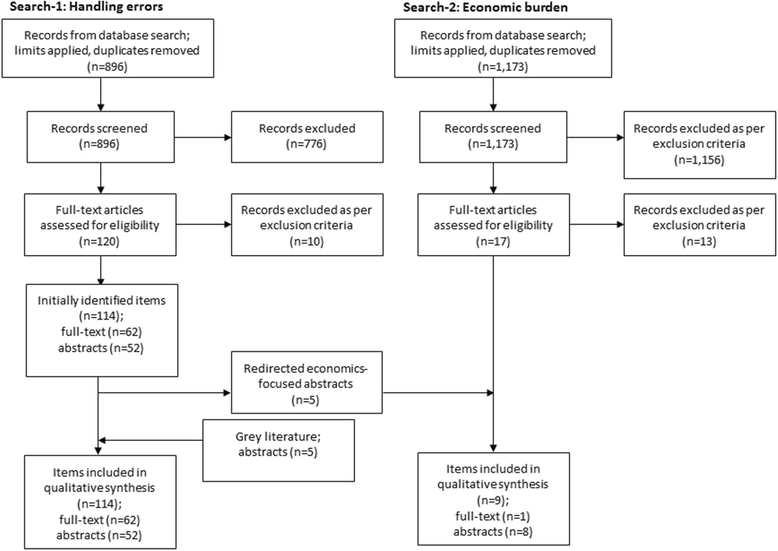
Methods: A systematic review was conducted to define 'critical' errors and their impact on health outcomes and resource use between 2004 and 2016, using key search terms for inhaler errors in asthma and COPD (Search-1) and associated health-economic and patient burden (Search-2).
Results: Search-1 identified 62 manuscripts, 47 abstracts, and 5 conference proceedings (n = 114 total). Search-2 identified 9 studies. We observed 299 descriptions of critical error. Age, education status, previous inhaler instruction, comorbidities and socioeconomic status were associated with worse handling error frequency. A significant association was found between inhaler errors and poor disease outcomes (exacerbations), and greater health-economic burden.
Conclusions: We have shown wide variations in how critical errors are defined, and the evidence shows an important association between inhaler errors and worsened health outcomes. Given the negative impact diminished disease outcomes impose on resource use, our findings highlight the importance of achieving optimal inhaler technique, and a need for a consensus on defining critical and non-critical errors.
Keywords: Adherence; Aerosols; Errors; Inhalers; Obstructive lung diseases.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
In the last 5 years, Omar S. Usmani and/or his department received research grants, unrestricted educational grants, and/or fees for lectures and advisory board meetings from Aerocrine, Astra Zeneca, Boehringer Ingelheim, Chiesi, Cipla, Edmond Pharma, GlaxoSmithKline, Napp, Mundipharma International, Prosonix, Sandoz, Takeda, Zentiva.
In the last 5 years, Federico Lavorini received fees for lectures and advisory board meetings from Astra Zeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini International, TEVA, Zentiva.
In the last 3 years, Richard Dekhuijzen and/or his department received research grants, unrestricted educational grants, and/or fees for lectures and advisory board meetings from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma International, Novartis, Takeda and Teva.
William Dunlop and Jonathan Marshall are employees of Mundipharma International Ltd., United Kingdom.
Emily Farrington and Louise Heron are employees of Adelphi Values Ltd., UK. Adelphi Values Ltd. received funding from Mundipharma International Ltd. to support this research.
The authors do not report any conflict of interest with regards to the contents of this study other than those stated.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Gregory KL, Elliott D, Dunne P. Guide to aerosol delivery devices for physicians, nurses, pharmacists and other health care professionals [https://www.aarc.org/wp-content/uploads/2014/08/aerosol_guide_pro.pdf] Accessed: 10 Nov 2015.
-
- Price D, Marshall J, Turner R. Inhaler use in five european countries: analysis of sales data from Q4 2005 to Q4 2011. Value Health. 2012;15:A-PMD92.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

