Patterning the gastrointestinal epithelium to confer regional-specific functions
- PMID: 29339095
- PMCID: PMC6615902
- DOI: 10.1016/j.ydbio.2018.01.006
Patterning the gastrointestinal epithelium to confer regional-specific functions
Abstract
The gastrointestinal (GI) tract, in simplest terms, can be described as an epithelial-lined muscular tube extending along the cephalocaudal axis from the oral cavity to the anus. Although the general architecture of the GI tract organs is conserved from end to end, the presence of different epithelial tissue structures and unique epithelial cell types within each organ enables each to perform the distinct digestive functions required for efficient nutrient assimilation. Spatiotemporal regulation of signaling pathways and downstream transcription factors controls GI epithelial morphogenesis during development to confer essential regional-specific epithelial structures and functions. Here, we discuss the fundamental functions of each GI tract organ and summarize the diversity of epithelial structures present along the cephalocaudal axis of the GI tract. Next, we discuss findings, primarily from genetic mouse models, that have defined the roles of key transcription factors during epithelial morphogenesis, including p63, SOX2, SOX15, GATA4, GATA6, HNF4A, and HNF4G. Additionally, we examine how the Hedgehog, WNT, and BMP signaling pathways contribute to defining unique epithelial features along the cephalocaudal axis of the GI tract. Lastly, we examine the molecular mechanisms controlling regionalized cytodifferentiation of organ-specific epithelial cell types within the GI tract, concentrating on the stomach and small intestine. The delineation of GI epithelial patterning mechanisms in mice has provided fundamental knowledge to guide the development and refinement of three-dimensional GI organotypic culture models such as those derived from directed differentiation of human pluripotent stem cells and those derived directly from human tissue samples. Continued examination of these pathways will undoubtedly provide vital insights into the mechanisms of GI development and disease and may afford new avenues for innovative tissue engineering and personalized medicine approaches to treating GI diseases.
Keywords: Cytodifferentiation; Gastrointestinal development; Gastrointestinal epithelium; Morphogenesis; Regionalization; Signaling pathways; Transcription factors.
Copyright © 2018. Published by Elsevier Inc.
Figures




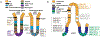
References
-
- Alaynick WA, Way JM, Wilson SA, Benson WG, Pei L, Downes M, Yu R, Jonker JW, Holt JA, Rajpal DK, Li H, Stuart J, McPherson R, Remlinger KS, Chang CY, McDonnell DP, Evans RM, Billin AN, 2010. ERRgamma regulates cardiac, gastric, and renal potassium homeostasis. Mol Endocrinol 24, 299–309. - PMC - PubMed
-
- Andreu P, Peignon G, Slomianny C, Taketo MM, Colnot S, Robine S, Lamarque D, Laurent-Puig P, Perret C, Romagnolo B, 2008. A genetic study of the role of the Wnt/beta-catenin signalling in Paneth cell differentiation. Dev Biol 324, 288–296. - PubMed
-
- Aubin J, Déry U, Lemieux M, Chailler P, Jeannotte L, 2002. Stomach regional specification requires Hoxa5-driven mesenchymal-epithelial signaling. Development 129, 4075–4087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

