Ocular delivery of proteins and peptides: Challenges and novel formulation approaches
- PMID: 29339145
- PMCID: PMC5995646
- DOI: 10.1016/j.addr.2018.01.008
Ocular delivery of proteins and peptides: Challenges and novel formulation approaches
Abstract
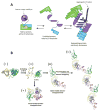
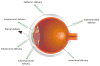
The impact of proteins and peptides on the treatment of various conditions including ocular diseases over the past few decades has been advanced by substantial breakthroughs in structural biochemistry, genetic engineering, formulation and delivery approaches. Formulation and delivery of proteins and peptides, such as monoclonal antibodies, aptamers, recombinant proteins and peptides to ocular tissues poses significant challenges owing to their large size, poor permeation and susceptibility to degradation. A wide range of advanced drug delivery systems including polymeric controlled release systems, cell-based delivery and nanowafers are being exploited to overcome the challenges of frequent administration to ocular tissues. The next generation systems integrated with new delivery technologies are anticipated to generate improved efficacy and safety through the expansion of the therapeutic target space. This review will highlight recent advances in formulation and delivery strategies of protein and peptide based biopharmaceuticals. We will also describe the current state of proteins and peptides based ocular therapy and future therapeutic opportunities.
Keywords: AMD; Barriers; Biologics; Biopharmaceuticals; Controlled release; Drug delivery; Eye; Macromolecules; Targeting.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures




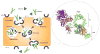



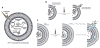


Similar articles
-
Stimuli-Responsive Polymeric Systems for Controlled Protein and Peptide Delivery: Future Implications for Ocular Delivery.Molecules. 2016 Jul 30;21(8):1002. doi: 10.3390/molecules21081002. Molecules. 2016. PMID: 27483234 Free PMC article. Review.
-
A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules.Biotechnol Adv. 2014 Nov 15;32(7):1269-1282. doi: 10.1016/j.biotechadv.2014.07.006. Epub 2014 Aug 3. Biotechnol Adv. 2014. PMID: 25099657 Review.
-
Recent perspectives on the delivery of biologics to back of the eye.Expert Opin Drug Deliv. 2017 May;14(5):631-645. doi: 10.1080/17425247.2016.1227783. Epub 2016 Sep 6. Expert Opin Drug Deliv. 2017. PMID: 27573097 Free PMC article. Review.
-
A review on parenteral delivery of peptides and proteins.Drug Dev Ind Pharm. 2019 Sep;45(9):1403-1420. doi: 10.1080/03639045.2019.1628770. Epub 2019 Jun 28. Drug Dev Ind Pharm. 2019. PMID: 31215293 Review.
-
Nanocarriers in ocular drug delivery: an update review.Curr Pharm Des. 2009;15(23):2724-50. doi: 10.2174/138161209788923886. Curr Pharm Des. 2009. PMID: 19689343 Review.
Cited by
-
Injectable PTHF-based thermogelling polyurethane implants for long-term intraocular application.Biomater Res. 2022 Dec 2;26(1):70. doi: 10.1186/s40824-022-00316-z. Biomater Res. 2022. PMID: 36461130 Free PMC article.
-
A Review of Techniques for Biodelivery of Nerve Growth Factor (NGF) to the Brain in Relation to Alzheimer's Disease.Adv Exp Med Biol. 2021;1331:167-191. doi: 10.1007/978-3-030-74046-7_11. Adv Exp Med Biol. 2021. PMID: 34453298 Review.
-
Application of Convergent Science and Technology toward Ocular Disease Treatment.Pharmaceuticals (Basel). 2023 Mar 16;16(3):445. doi: 10.3390/ph16030445. Pharmaceuticals (Basel). 2023. PMID: 36986546 Free PMC article. Review.
-
Multifunctional nano-in-micro delivery systems for targeted therapy in fundus neovascularization diseases.J Nanobiotechnology. 2024 Jun 20;22(1):354. doi: 10.1186/s12951-024-02614-1. J Nanobiotechnology. 2024. PMID: 38902775 Free PMC article. Review.
-
Sirolimus-Loaded Intravitreal Implant for Effective Treatment of Experimental Uveitis.AAPS PharmSciTech. 2021 Jan 6;22(1):35. doi: 10.1208/s12249-020-01898-4. AAPS PharmSciTech. 2021. PMID: 33404988
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

