Cardiac macrophages promote diastolic dysfunction
- PMID: 29339450
- PMCID: PMC5789416
- DOI: 10.1084/jem.20171274
Cardiac macrophages promote diastolic dysfunction
Abstract
Macrophages populate the healthy myocardium and, depending on their phenotype, may contribute to tissue homeostasis or disease. Their origin and role in diastolic dysfunction, a hallmark of cardiac aging and heart failure with preserved ejection fraction, remain unclear. Here we show that cardiac macrophages expand in humans and mice with diastolic dysfunction, which in mice was induced by either hypertension or advanced age. A higher murine myocardial macrophage density results from monocyte recruitment and increased hematopoiesis in bone marrow and spleen. In humans, we observed a parallel constellation of hematopoietic activation: circulating myeloid cells are more frequent, and splenic 18F-FDG PET/CT imaging signal correlates with echocardiographic indices of diastolic dysfunction. While diastolic dysfunction develops, cardiac macrophages produce IL-10, activate fibroblasts, and stimulate collagen deposition, leading to impaired myocardial relaxation and increased myocardial stiffness. Deletion of IL-10 in macrophages improves diastolic function. These data imply expansion and phenotypic changes of cardiac macrophages as therapeutic targets for cardiac fibrosis leading to diastolic dysfunction.
© 2018 Hulsmans et al.
Figures
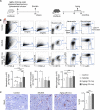



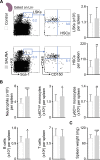
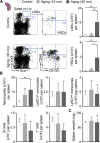


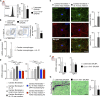
Comment in
-
Interleukin-10 stiffens the heart.J Exp Med. 2018 Feb 5;215(2):379-381. doi: 10.1084/jem.20180049. Epub 2018 Jan 18. J Exp Med. 2018. PMID: 29348198 Free PMC article.
-
Heart failure: Macrophages promote cardiac fibrosis and diastolic dysfunction.Nat Rev Cardiol. 2018 Apr;15(4):196-197. doi: 10.1038/nrcardio.2018.19. Epub 2018 Mar 1. Nat Rev Cardiol. 2018. PMID: 29493572 No abstract available.
References
-
- Ather S., Chan W., Bozkurt B., Aguilar D., Ramasubbu K., Zachariah A.A., Wehrens X.H., and Deswal A.. 2012. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J. Am. Coll. Cardiol. 59:998–1005. 10.1016/j.jacc.2011.11.040 - DOI - PMC - PubMed
-
- Beerman I., Bhattacharya D., Zandi S., Sigvardsson M., Weissman I.L., Bryder D., and Rossi D.J.. 2010. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. USA. 107:5465–5470. 10.1073/pnas.1000834107 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

