The Bardet-Biedl syndrome protein complex is an adapter expanding the cargo range of intraflagellar transport trains for ciliary export
- PMID: 29339469
- PMCID: PMC5798339
- DOI: 10.1073/pnas.1713226115
The Bardet-Biedl syndrome protein complex is an adapter expanding the cargo range of intraflagellar transport trains for ciliary export
Abstract
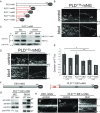
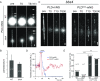
Bardet-Biedl syndrome (BBS) is a ciliopathy resulting from defects in the BBSome, a conserved protein complex. BBSome mutations affect ciliary membrane composition, impairing cilia-based signaling. The mechanism by which the BBSome regulates ciliary membrane content remains unknown. Chlamydomonas bbs mutants lack phototaxis and accumulate phospholipase D (PLD) in the ciliary membrane. Single particle imaging revealed that PLD comigrates with BBS4 by intraflagellar transport (IFT) while IFT of PLD is abolished in bbs mutants. BBSome deficiency did not alter the rate of PLD entry into cilia. Membrane association and the N-terminal 58 residues of PLD are sufficient and necessary for BBSome-dependent transport and ciliary export. The replacement of PLD's ciliary export sequence (CES) caused PLD to accumulate in cilia of cells with intact BBSomes and IFT. The buildup of PLD inside cilia impaired phototaxis, revealing that PLD is a negative regulator of phototactic behavior. We conclude that the BBSome is a cargo adapter ensuring ciliary export of PLD on IFT trains to regulate phototaxis.
Keywords: BBSome; cilia; ciliopathy; flagella; phospholipase D.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

