Ebola virus proteins NP, VP35, and VP24 are essential and sufficient to mediate nucleocapsid transport
- PMID: 29339477
- PMCID: PMC5798334
- DOI: 10.1073/pnas.1712263115
Ebola virus proteins NP, VP35, and VP24 are essential and sufficient to mediate nucleocapsid transport
Abstract
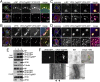
The intracytoplasmic movement of nucleocapsids is a crucial step in the life cycle of enveloped viruses. Determination of the viral components necessary for viral nucleocapsid transport competency is complicated by the dynamic and complex nature of nucleocapsid assembly and the lack of appropriate model systems. Here, we established a live-cell imaging system based on the ectopic expression of fluorescent Ebola virus (EBOV) fusion proteins, allowing the visualization and analysis of the movement of EBOV nucleocapsid-like structures with different protein compositions. Only three of the five EBOV nucleocapsid proteins-nucleoprotein, VP35, and VP24-were necessary and sufficient to form transport-competent nucleocapsid-like structures. The transport of these structures was found to be dependent on actin polymerization and to have dynamics that were undistinguishable from those of nucleocapsids in EBOV-infected cells. The intracytoplasmic movement of nucleocapsid-like structures was completely independent of the viral matrix protein VP40 and the viral surface glycoprotein GP. However, VP40 greatly enhanced the efficiency of nucleocapsid recruitment into filopodia, the sites of EBOV budding.
Keywords: Ebola virus; live-cell imaging; nucleocapsid; replicon system; transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Sodeik B. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 2000;8:465–472. - PubMed
-
- Smith GA, Enquist LW. Break ins and break outs: Viral interactions with the cytoskeleton of mammalian cells. Annu Rev Cell Dev Biol. 2002;18:135–161. - PubMed
-
- Goley ED, et al. Dynamic nuclear actin assembly by Arp2/3 complex and a baculovirus WASP-like protein. Science. 2006;314:464–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

