Infected erythrocytes expressing DC13 PfEMP1 differ from recombinant proteins in EPCR-binding function
- PMID: 29339517
- PMCID: PMC5798336
- DOI: 10.1073/pnas.1712879115
Infected erythrocytes expressing DC13 PfEMP1 differ from recombinant proteins in EPCR-binding function
Abstract
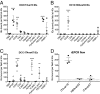
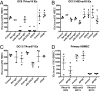
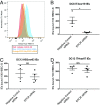
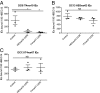
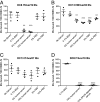
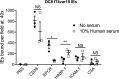
Recent advances have identified a new paradigm for cerebral malaria pathogenesis in which endothelial protein C receptor (EPCR) is a major host receptor for sequestration of Plasmodium falciparum-infected erythrocytes (IEs) in the brain and other vital organs. The parasite adhesins that bind EPCR are members of the IE variant surface antigen family Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) containing specific adhesion domains called domain cassette (DC) 8 and DC13. The binding interaction site between PfEMP1 and EPCR has been mapped by biophysical and crystallography studies using recombinant proteins. However, studies examining the interaction of native PfEMP1 on the IE surface with EPCR are few. We aimed to study binding to EPCR by IEs expressing DC8 and DC13 PfEMP1 variants whose recombinant proteins have been used in key prior functional and structural studies. IE binding to EPCR immobilized on plastic and on human brain endothelial cells was examined in static and flow adhesion assays. Unexpectedly, we found that IEs expressing the DC13 PfEMP1 variant HB3var03 or IT4var07 did not bind to EPCR on plastic and the binding of these variants to brain endothelial cells was not dependent on EPCR. IEs expressing the DC8 variant IT4var19 did bind to EPCR, but this interaction was inhibited if normal human serum or plasma was present, raising the possibility that IE-EPCR interaction may be prevented by plasma components under physiological conditions. These data highlight a discrepancy in EPCR-binding activity between PfEMP1 recombinant proteins and IEs, and indicate the critical need for further research to understand the pathophysiological significance of the PfEMP1-EPCR interaction.
Keywords: EPCR; PfEMP1; cell adhesion; endothelium; malaria.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Marchiafava E, Bignami A. On Summer-Autumnal Fever. The New Sydenham Society; London: 1894.
-
- Baruch DI, et al. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: Conserved function with variant sequence. Blood. 1997;90:3766–3775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

