Mechanisms of redox metabolism and cancer cell survival during extracellular matrix detachment
- PMID: 29339552
- PMCID: PMC5961063
- DOI: 10.1074/jbc.TM117.000260
Mechanisms of redox metabolism and cancer cell survival during extracellular matrix detachment
Abstract
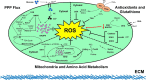
Nontransformed cells that become detached from the extracellular matrix (ECM) undergo dysregulation of redox homeostasis and cell death. In contrast, cancer cells often acquire the ability to mitigate programmed cell death pathways and recalibrate the redox balance to survive after ECM detachment, facilitating metastatic dissemination. Accordingly, recent studies of the mechanisms by which cancer cells overcome ECM detachment-induced metabolic alterations have focused on mechanisms in redox homeostasis. The insights into these mechanisms may inform the development of therapeutics that manipulate redox homeostasis to eliminate ECM-detached cancer cells. Here, we review how ECM-detached cancer cells balance redox metabolism for survival.
Keywords: anoikis; apoptosis; cancer; cell death; cell metabolism; extracellular matrix; reactive oxygen species (ROS); redox regulation.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

