The redox requirements of proliferating mammalian cells
- PMID: 29339555
- PMCID: PMC5961062
- DOI: 10.1074/jbc.TM117.000239
The redox requirements of proliferating mammalian cells
Abstract
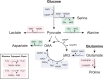
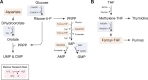
Cell growth and division require nutrients, and proliferating cells use a variety of sources to acquire the amino acids, lipids, and nucleotides that support macromolecule synthesis. Lipids are more reduced than other nutrients, whereas nucleotides and amino acids are typically more oxidized. Cells must therefore generate reducing and oxidizing (redox) equivalents to convert consumed nutrients into biosynthetic precursors. To that end, redox cofactor metabolism plays a central role in meeting cellular redox requirements. In this Minireview, we highlight the biosynthetic pathways that involve redox reactions and discuss their integration with metabolism in proliferating mammalian cells.
Keywords: cell metabolism; cell proliferation; metabolism; mitochondrial metabolism; oxidation-reduction (redox).
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Wheaton W. W., Weinberg S. E., Hamanaka R. B., Soberanes S., Sullivan L. B., Anso E., Glasauer A., Dufour E., Mutlu G. M., Budigner G. S., and Chandel N. S. (2014) Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 3, e02242 10.7554/eLife.02242 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

