Phenotype and Functional Features of Human Telomerase Reverse Transcriptase Immortalized Human Airway Smooth Muscle Cells from Asthmatic and Non-Asthmatic Donors
- PMID: 29339735
- PMCID: PMC5770384
- DOI: 10.1038/s41598-017-18429-0
Phenotype and Functional Features of Human Telomerase Reverse Transcriptase Immortalized Human Airway Smooth Muscle Cells from Asthmatic and Non-Asthmatic Donors
Abstract
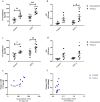
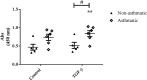
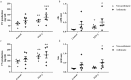
Asthma is an obstructive respiratory disease characterised by chronic inflammation with airway hyperresponsiveness. In asthmatic airways, there is an increase in airway smooth muscle (ASM) cell bulk, which differs from non-asthmatic ASM in characteristics. This study aimed to assess the usefulness of hTERT immortalisation of human ASM cells as a research tool. Specifically we compared proliferative capacity, inflammatory mediator release and extracellular matrix (ECM) production in hTERT immortalised and parent primary ASM cells from asthmatic and non-asthmatic donors. Our studies revealed no significant differences in proliferation, IL-6 and eotaxin-1 production, or CTGF synthesis between donor-matched parent and hTERT immortalised ASM cell lines. However, deposition of ECM proteins fibronectin and fibulin-1 was significantly lower in immortalised ASM cells compared to corresponding primary cells. Notably, previously reported differences in proliferation and inflammatory mediator release between asthmatic and non-asthmatic ASM cells were retained, but excessive ECM protein deposition in asthmatic ASM cells was lost in hTERT ASM cells. This study shows that hTERT immortalised ASM cells mirror primary ASM cells in proliferation and inflammatory profile characteristics. Moreover, we demonstrate both strengths and weaknesses of this immortalised cell model as a representation of primary ASM cells for future asthma pathophysiological research.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

