A Single Dose of Modified Vaccinia Ankara expressing Ebola Virus Like Particles Protects Nonhuman Primates from Lethal Ebola Virus Challenge
- PMID: 29339750
- PMCID: PMC5770434
- DOI: 10.1038/s41598-017-19041-y
A Single Dose of Modified Vaccinia Ankara expressing Ebola Virus Like Particles Protects Nonhuman Primates from Lethal Ebola Virus Challenge
Abstract
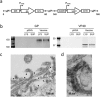
Ebola virus (EBOV), isolate Makona, was the causative agent of the West African epidemic devastating predominantly Guinea, Liberia and Sierra Leone from 2013-2016. While several experimental vaccine and treatment approaches have been accelerated through human clinical trials, there is still no approved countermeasure available against this disease. Here, we report the construction and preclinical efficacy testing of a novel recombinant modified vaccinia Ankara (MVA)-based vaccine expressing the EBOV-Makona glycoprotein GP and matrix protein VP40 (MVA-EBOV). GP and VP40 form EBOV-like particles and elicit protective immune responses. In this study, we report 100% protection against lethal EBOV infection in guinea pigs after prime/boost vaccination with MVA-EBOV. Furthermore, this MVA-EBOV protected macaques from lethal disease after a single dose or prime/boost vaccination. The vaccine elicited a variety of antibody responses to both antigens, including neutralizing antibodies and antibodies with antibody-dependent cellular cytotoxic activity specific for GP. This is the first report that a replication-deficient MVA vector can confer full protection against lethal EBOV challenge after a single dose vaccination in macaques.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- CDC. Outbreaks Chronology: Ebola Virus Disease, http://www.cdc.gov/vhf/ebola/outbreaks/history/chronology.html (2015).
-
- CDC. Chronology of Marburg Hemorrhagic Fever Outbreaks, http://www.cdc.gov/vhf/marburg/resources/outbreak-table.html (2015).
-
- WHO. WHO Ebola Situation Report (2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

