Evaluation of green tea extract as a safe personal hygiene against viral infections
- PMID: 29339972
- PMCID: PMC5759362
- DOI: 10.1186/s13036-017-0092-1
Evaluation of green tea extract as a safe personal hygiene against viral infections
Abstract
Background: Viral infections often pose tremendous public health concerns as well as economic burdens. Despite the availability of vaccines or antiviral drugs, personal hygiene is considered as effective means as the first-hand measure against viral infections. The green tea catechins, in particular, epigallocatechin-3-gallate (EGCG), are known to exert potent antiviral activity. In this study, we evaluated the green tea extract as a safe personal hygiene against viral infections.
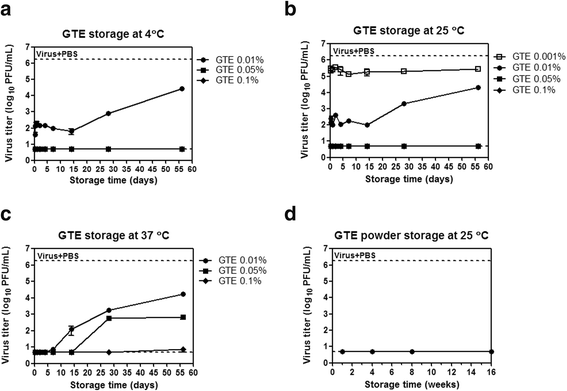
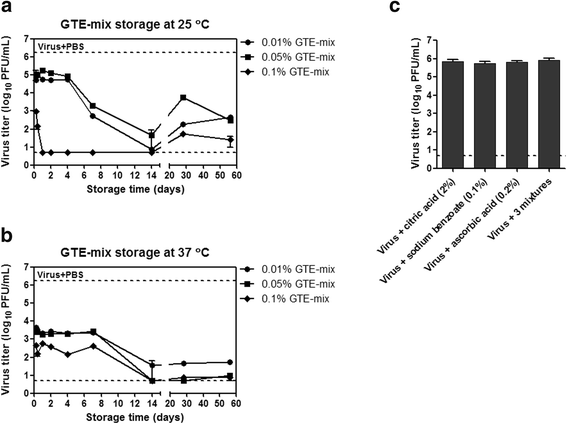
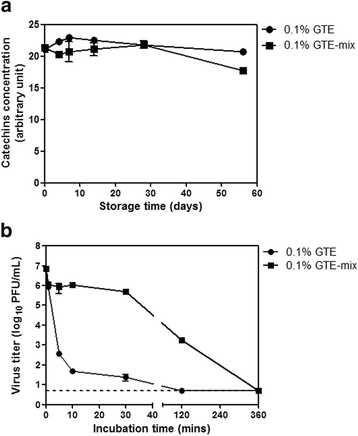
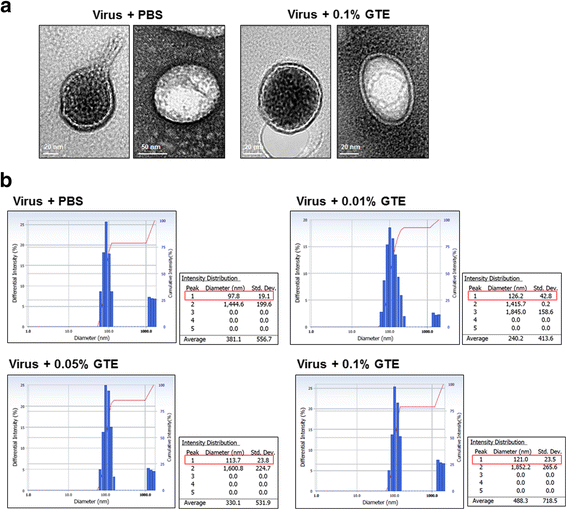
Results: Using the influenza virus A/Puerto Rico/8/34 (H1N1) as a model, we examined the duration of the viral inactivating activity of green tea extract (GTE) under prolonged storage at various temperature conditions. Even after the storage for 56 days at different temperatures, 0.1% GTE completely inactivated 106 PFU of the virus (6 log10 reduction), and 0.01% and 0.05% GTE resulted in 2 log10 reduction of the viral titers. When supplemented with 2% citric acid, 0.1% sodium benzoate, and 0.2% ascorbic acid as anti-oxidant, the inactivating activity of GTE was temporarily compromised during earlier times of storage. However, the antiviral activity of the GTE was steadily recovered up to similar levels with those of the same concentrations of GTE without the supplements, effectively prolonging the duration of the virucidal function over extended period. Cryo-EM and DLS analyses showed a slight increase in the overall size of virus particles by GTE treatment. The results suggest that the virucidal activity of GTE is mediated by oxidative crosslinking of catechins to the viral proteins and the change of physical properties of viral membranes.
Conclusions: The durability of antiviral effects of GTE was examined as solution type and powder types over extended periods at various temperature conditions using human influenza A/H1N1 virus. GTE with supplements demonstrated potent viral inactivating activity, resulting in greater than 4 log10 reduction of viral titers even after storage for up to two months at a wide range of temperatures. These data suggest that GTE-based antiviral agents could be formulated as a safe and environmentally friendly personal hygiene against viral infections.
Keywords: Antioxidant; Antiviral; Catechins; Green tea extract; Influenza virus.
Conflict of interest statement
Not applicable.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Preparation and antioxidant activity of green tea extract enriched in epigallocatechin (EGC) and epigallocatechin gallate (EGCG).J Agric Food Chem. 2009 Feb 25;57(4):1349-53. doi: 10.1021/jf803143n. J Agric Food Chem. 2009. PMID: 19182914
-
Green tea extract reduces viral proliferation and ROS production during Feline Herpesvirus type-1 (FHV-1) infection.BMC Vet Res. 2024 Aug 22;20(1):374. doi: 10.1186/s12917-024-04227-0. BMC Vet Res. 2024. PMID: 39175036 Free PMC article.
-
Green tea extract containing enhanced levels of epimerized catechins attenuates scopolamine-induced memory impairment in mice.J Ethnopharmacol. 2020 Aug 10;258:112923. doi: 10.1016/j.jep.2020.112923. Epub 2020 May 1. J Ethnopharmacol. 2020. PMID: 32360798
-
Salubrious Effects of Green Tea Catechins on Fatty Liver Disease: A Systematic Review.Medicines (Basel). 2022 Mar 1;9(3):20. doi: 10.3390/medicines9030020. Medicines (Basel). 2022. PMID: 35323719 Free PMC article. Review.
-
Safety assessment of green tea based beverages and dried green tea extracts as nutritional supplements.Toxicol Lett. 2017 Aug 5;277:104-108. doi: 10.1016/j.toxlet.2017.06.008. Epub 2017 Jun 24. Toxicol Lett. 2017. PMID: 28655517 Review.
Cited by
-
Evaluation of medicinal herbs as a potential therapeutic option against SARS-CoV-2 targeting its main protease.Phytother Res. 2020 Dec;34(12):3411-3419. doi: 10.1002/ptr.6802. Epub 2020 Aug 4. Phytother Res. 2020. PMID: 32748969 Free PMC article.
-
Environment signal dependent biocontainment systems for engineered organisms: Leveraging triggered responses and combinatorial systems.Synth Syst Biotechnol. 2024 Dec 20;10(2):356-364. doi: 10.1016/j.synbio.2024.12.005. eCollection 2025 Jun. Synth Syst Biotechnol. 2024. PMID: 39830078 Free PMC article. Review.
-
Effect of green tea and mulberry leaf powders on the gut microbiota of chicken.BMC Vet Res. 2019 Mar 6;15(1):77. doi: 10.1186/s12917-019-1822-z. BMC Vet Res. 2019. PMID: 30841884 Free PMC article.
-
Quality characteristics of infusion and health consequences: a comparative study between orthodox and CTC green teas.RSC Adv. 2020 Sep 3;10(54):32833-32842. doi: 10.1039/d0ra06254e. eCollection 2020 Sep 1. RSC Adv. 2020. PMID: 35516505 Free PMC article.
-
Characterization of the Trans-Epithelial Transport of Green Tea (C. sinensis) Catechin Extracts with In Vitro Inhibitory Effect against the SARS-CoV-2 Papain-like Protease Activity.Molecules. 2021 Nov 8;26(21):6744. doi: 10.3390/molecules26216744. Molecules. 2021. PMID: 34771162 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

