Prexasertib, a cell cycle checkpoint kinases 1 and 2 inhibitor, increases in vitro toxicity of PARP inhibition by preventing Rad51 foci formation in BRCA wild type high-grade serous ovarian cancer
- PMID: 29340034
- PMCID: PMC5762302
- DOI: 10.18632/oncotarget.22195
Prexasertib, a cell cycle checkpoint kinases 1 and 2 inhibitor, increases in vitro toxicity of PARP inhibition by preventing Rad51 foci formation in BRCA wild type high-grade serous ovarian cancer
Abstract
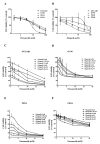
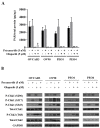
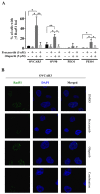
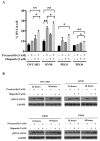
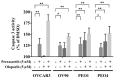
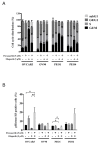
PARP inhibitors (PARPi) have been effective in high-grade serous ovarian cancer (HGSOC), although clinical activity is limited against BRCA wild type HGSOC. The nearly universal loss of normal p53 regulation in HGSOCs causes dysfunction in the G1/S checkpoint, making tumor cells reliant on Chk1-mediated G2/M cell cycle arrest for DNA repair. Therefore, Chk1 is a reasonable target for a combination strategy with PARPi in treating BRCA wild type HGSOC. Here we investigated the combination of prexasertib mesylate monohydrate (LY2606368), a Chk1 and Chk2 inhibitor, and a PARP inhibitor, olaparib, in HGSOC cell lines (OVCAR3, OV90, PEO1 and PEO4) using clinically attainable concentrations. Our findings showed combination treatment synergistically decreased cell viability in all cell lines and induced greater DNA damage and apoptosis than the control and/or monotherapies (p<0.05). Treatment with olaparib in BRCA wild type HGSOC cells caused formation of Rad51 foci, whereas the combination treatment with prexasertib inhibited transnuclear localization of Rad51, a key protein in homologous recombination repair. Overall, our data provide evidence that prexasertib and olaparib combination resulted in synergistic cytotoxic effects against BRCA wild type HGSOC cells through reduced Rad51 foci formation and greater induction of apoptosis. This may be a novel therapeutic strategy for HGSOC.
Keywords: LY2606368; PARP inhibitor; cell cycle checkpoint kinase inhibitor; olaparib; prexasertib.
Conflict of interest statement
CONFLICTS OF INTEREST The authors disclose no potential conflicts of interest.
Figures
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. - PubMed
-
- Maringe C, Walters S, Butler J, Coleman MP, Hacker N, Hanna L, Mosgaard BJ, Nordin A, Rosen B, Engholm G, Gjerstorff ML, Hatcher J, Johannesen TB, et al. ICBP Module 1 Working Group Stage at diagnosis and ovarian cancer survival: evidence from the International Cancer Benchmarking Partnership. Gynecol Oncol. 2012;127:75–82. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

