Indomethacin-based stimuli-responsive micelles combined with paclitaxel to overcome multidrug resistance
- PMID: 29340053
- PMCID: PMC5762321
- DOI: 10.18632/oncotarget.22781
Indomethacin-based stimuli-responsive micelles combined with paclitaxel to overcome multidrug resistance
Retraction in
-
Retraction: Indomethacin-based stimuli-responsive micelles combined with paclitaxel to overcome multidrug resistance.Oncotarget. 2025 Jul 9;16:559. doi: 10.18632/oncotarget.28753. Oncotarget. 2025. PMID: 40784035 Free PMC article. No abstract available.
Abstract
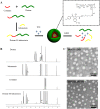
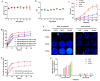
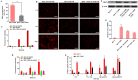
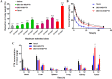
Development of multidrug resistance against antitumor agents is a major limiting factor for the successful chemotherapy. Currently, both amphiphilic polymeric micelles and chemosensitizers have been proposed to overcome MDR during chemotherapy. Herein, the redox-responsive polymeric micelles composed of dextran and indomethacin (as chemosensitizer) using a disulfide bond as the linker are prepared (DEX-SS-IND) for delivery of antitumor agent paclitaxel (PTX). The high level of glutathione in tumor cells selectively breaks the disulfide bond, leading to the rapid breakdown and deformation of redox-responsive polymeric micelles. The data show that DEX-SS-IND can spontaneously form the stable micelles with high loading content (9.48 ± 0.41%), a favorable size of 45 nm with a narrow polydispersity (0.157), good stability, and glutathione-triggered drug release behavior due to the rapid breakdown of disulfide bond between DEX and IND. In vitro antitumor assay shows DEX-SS-IND/PTX micelles effectively inhibit the proliferation of PTX-resistant breast cancer (MCF-7/PTX) cells. More impressively, DEX-SS-IND/PTX micelles possess the improved plasma pharmacokinetics, enhanced antitumor efficacy on tumor growth in the xenograft models of MCF-7/PTX cells, and better in vivo safety. Overall, DEX-SS-IND/PTX micelles display a great potential for cancer treatment, especially for multidrug resistance tumors.
Keywords: breast cancer; drug delivery system; indomethacin; multidrug resistance; paclitaxel.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no competing financial interest.
Figures

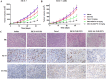
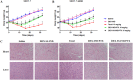
References
-
- Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. https//doi.org/10.1038/nrd1984. - DOI - PubMed
-
- Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–757. https//doi.org/10.1038/nature05630. - DOI - PubMed
-
- Zhang D, Kong YY, Sun JH, Huo SJ, Zhou M, Gui YL, Mu X, Chen H, Yu SQ, Xu Q. Co-delivery nanoparticles with characteristics of intracellular precision release drugs for overcoming multidrug resistance. Int J Nanomedicine. 2017;12:2081–2108. https//doi.org/10.2147/IJN.S128790. - DOI - PMC - PubMed
-
- Cheng T, Liu J, Ren J, Huang F, Ou H, Ding Y, Zhang Y, Ma R, An Y, Liu J, Shi L. Green tea catechin-based complex micelles combined with doxorubicin to overcome cardiotoxicity and multidrug resistance. Theranostics. 2016;6:1277–1292. https//doi.org/10.7150/thno.15133. - DOI - PMC - PubMed
-
- Yuan Y, Cai T, Xia X, Zhang R, Chiba P, Cai Y. Nanoparticle delivery of anticancer drugs overcomes multidrug resistance in breast cancer. Drug Deliv. 2016;23:3350–3357. https//doi.org/10.1080/10717544.2016.1178825. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

