Febuxostat attenuates ER stress mediated kidney injury in a rat model of hyperuricemic nephropathy
- PMID: 29340054
- PMCID: PMC5762322
- DOI: 10.18632/oncotarget.22784
Febuxostat attenuates ER stress mediated kidney injury in a rat model of hyperuricemic nephropathy
Abstract
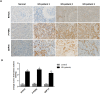
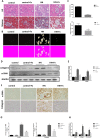
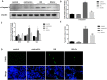
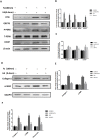
Hyperuricemia contributes to kidney tubular injury and kidney fibrosis. However, the underlying mechanism remains unclear. Here we examined the role of RTN1A, a novel endoplasmic reticulum (ER)-associated protein and ER stress in hyperuricemic nephropathy. We first found the expression of RTN1A and ER stress markers was significantly increased in kidney biopsies of hyperuricemia patients with kidney injury. In a rat model of hyperuricemic nephropathy (HN) established by oral administration of a mixture of adenine and potassium oxonate, increased expression of RTN1A and ER stress was shown in tubular and interstitial compartment of rat kidneys. Treatment of Febuxostat, a new selective inhibitor of xanthine oxidase (XO), not only attenuated renal tubular injury and tubulointerstitial fibrosis, but also reduced uric acid crystals deposition in HN rat kidneys. In vitro, Febuxostat also reduced ER stress and apoptosis in uric acid treated tubular epithelial cells. Our data suggest that RTN1A and ER stress mediate tubular cell injury and kidney fibrosis in HN. Urate-lowering therapy (ULT) with Febuxostat attenuates uric-acid induced ER stress in renal tubular cells and the progression of HN.
Keywords: ER stress; Febuxostat; hyperuricemia; hyperuricemic nephropathy; renal tubular cells.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that there are no conflicts of interest.
Figures



References
-
- Sanchez-Lozada LG, Tapia E, Lopez-Molina R, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M. Effects of acute and chronic L-arginine treatment in experimental hyperuricemia. Am J Physiol Renal Physiol. 2007;292:F1238–F1244. - PubMed
-
- Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. - PubMed
-
- Long CL, Qin XC, Pan ZY, Chen K, Zhang YF, Cui WY, Liu GS, Wang H. Activation of ATP-sensitive potassium channels protects vascular endothelial cells from hypertension and renal injury induced by hyperuricemia. J Hypertens. 2008;26:2326–2338. - PubMed
-
- Gude D, Chennamsetty S, Jha R. Fathoming uric acid nephropathy. Saudi J Kidney Dis Transpl. 2013;24:1259–1261. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

