Membrane Position Dependency of the pKa and Conductivity of the Protein Ion Channel
- PMID: 29340712
- PMCID: PMC6030496
- DOI: 10.1007/s00232-018-0013-3
Membrane Position Dependency of the pKa and Conductivity of the Protein Ion Channel
Abstract
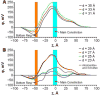
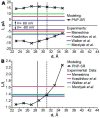
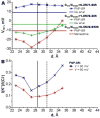
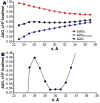
The dependency of current-voltage characteristics of the α-hemolysin channel on the channel position within the membrane was studied using Poisson-Nernst-Planck theory of ion conductivity with soft repulsion between mobile ions and protein atoms (SP-PNP). The presence of the membrane environment also influences the protonation state of the residues at the boundary of the water-lipid interface. In this work, we predict that Asp and Lys residues at the protein rim change their protonation state upon penetration to the lipid environment. Free energies of protein insertion in the membrane for different penetration depths were estimated using the Poisson-Boltzmann/solvent-accessible surface area (PB/SASA) model. The results show that rectification and reversal potentials are very sensitive to the relative position of channel in the membrane, which in turn contributes to alternative protonation states of lipid-penetrating ionizable groups. The prediction of channel position based on the matching of calculated rectification with experimentally determined rectification is in good agreement with recent neutron reflection experiments. Based on the results, we conclude that α-hemolysin membrane position is determined by a combination of factors and not only by the pattern of the surface hydrophobicity as is typically assumed.
Keywords: Current–voltage characteristics; Ion channels; Ion conductivity; Poisson–Nernst–Plank theory; pKa in membrane; α-Hemolysin.
Figures

Similar articles
-
Soft wall ion channel in continuum representation with application to modeling ion currents in α-hemolysin.J Phys Chem B. 2010 Nov 25;114(46):15180-90. doi: 10.1021/jp1046062. Epub 2010 Oct 28. J Phys Chem B. 2010. PMID: 21028776 Free PMC article.
-
Ion permeation through the alpha-hemolysin channel: theoretical studies based on Brownian dynamics and Poisson-Nernst-Plank electrodiffusion theory.Biophys J. 2004 Oct;87(4):2299-309. doi: 10.1529/biophysj.104.044008. Biophys J. 2004. PMID: 15454431 Free PMC article.
-
Imaging alpha-hemolysin with molecular dynamics: ionic conductance, osmotic permeability, and the electrostatic potential map.Biophys J. 2005 Jun;88(6):3745-61. doi: 10.1529/biophysj.104.058727. Epub 2005 Mar 11. Biophys J. 2005. PMID: 15764651 Free PMC article.
-
Theoretical simulation of the ion current rectification (ICR) in nano-pores based on the Poisson-Nernst-Planck (PNP) model.Phys Chem Chem Phys. 2014 Jan 7;16(1):23-32. doi: 10.1039/c3cp51712h. Phys Chem Chem Phys. 2014. PMID: 24253284 Review.
-
The interpretation of membrane current voltage relations: a Nernst-Planck analysis.Prog Biophys Mol Biol. 1978;34(2):81-107. doi: 10.1016/0079-6107(79)90015-4. Prog Biophys Mol Biol. 1978. PMID: 375300 Review. No abstract available.
Cited by
-
Lipid Membranes and Reactions at Lipid Interfaces: Theory, Experiments, and Applications.J Membr Biol. 2018 Jun;251(3):295-298. doi: 10.1007/s00232-018-0039-6. Epub 2018 Jun 29. J Membr Biol. 2018. PMID: 29959445 No abstract available.
References
-
- Hille B. Ion Channels of Excitable Membranes. Sinauer Associates; 2001. 3rd Casebound edition 2001.
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. Garland Science; 2007. 2007.
-
- Kasianowicz JJ, Robertson JWF, Chan ER, Reiner JE, Stanford VM. Nanoscopic Porous Sensors. Annual Review of Analytical Chemistry. 2008;1(1):737–766. - PubMed
-
- Branton D, Deamer DW, Marziali A, Bayley H, Benner SA, Butler T, Di Ventra M, Garaj S, Hibbs A, Huang X, Jovanovich SB, Krstic PS, Lindsay S, Ling XS, Mastrangelo CH, Meller A, Oliver JS, Pershin YV, Ramsey JM, Riehn R, Soni GV, Tabard-Cossa V, Wanunu M, Wiggin M, Schloss JA. The potential and challenges of nanopore sequencing. Nat Biotech. 2008;26(10):1146–1153. - PMC - PubMed
-
- Eisenberg RS. From Structure to Function in Open Ionic Channels. J Membr Biol. 1999;171(1):1–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

