Global versus local mechanisms of temperature sensing in ion channels
- PMID: 29340775
- PMCID: PMC5945320
- DOI: 10.1007/s00424-017-2102-z
Global versus local mechanisms of temperature sensing in ion channels
Abstract
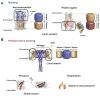
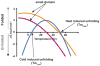
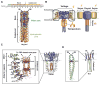
Ion channels turn diverse types of inputs, ranging from neurotransmitters to physical forces, into electrical signals. Channel responses to ligands generally rely on binding to discrete sensor domains that are coupled to the portion of the channel responsible for ion permeation. By contrast, sensing physical cues such as voltage, pressure, and temperature arises from more varied mechanisms. Voltage is commonly sensed by a local, domain-based strategy, whereas the predominant paradigm for pressure sensing employs a global response in channel structure to membrane tension changes. Temperature sensing has been the most challenging response to understand and whether discrete sensor domains exist for pressure and temperature has been the subject of much investigation and debate. Recent exciting advances have uncovered discrete sensor modules for pressure and temperature in force-sensitive and thermal-sensitive ion channels, respectively. In particular, characterization of bacterial voltage-gated sodium channel (BacNaV) thermal responses has identified a coiled-coil thermosensor that controls channel function through a temperature-dependent unfolding event. This coiled-coil thermosensor blueprint recurs in other temperature sensitive ion channels and thermosensitive proteins. Together with the identification of ion channel pressure sensing domains, these examples demonstrate that "local" domain-based solutions for sensing force and temperature exist and highlight the diversity of both global and local strategies that channels use to sense physical inputs. The modular nature of these newly discovered physical signal sensors provides opportunities to engineer novel pressure-sensitive and thermosensitive proteins and raises new questions about how such modular sensors may have evolved and empowered ion channel pores with new sensibilities.
Keywords: BacNav; Bacterial voltage gated sodium channel; Coiled-coil; Heat capacity; Ion channel; TRP channels; Temperature sensing; ΔCp.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

