Minimizing inequality in access to precision medicine in breast cancer by real-time population-based molecular analysis in the SCAN-B initiative
- PMID: 29341157
- PMCID: PMC5817401
- DOI: 10.1002/bjs.10741
Minimizing inequality in access to precision medicine in breast cancer by real-time population-based molecular analysis in the SCAN-B initiative
Abstract
Background: Selection of systemic therapy for primary breast cancer is currently based on clinical biomarkers along with stage. Novel genomic tests are continuously being introduced as more precise tools for guidance of therapy, although they are often developed for specific patient subgroups. The Sweden Cancerome Analysis Network - Breast (SCAN-B) initiative aims to include all patients with breast cancer for tumour genomic analysis, and to deliver molecular subtype and mutational data back to the treating physician.
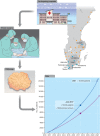
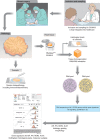
Methods: An infrastructure for collection of blood and fresh tumour tissue from all patients newly diagnosed with breast cancer was set up in 2010, initially including seven hospitals within the southern Sweden regional catchment area, which has 1.8 million inhabitants. Inclusion of patients was implemented into routine clinical care, with collection of tumour tissue at local pathology departments for transport to the central laboratory, where routines for rapid sample processing, RNA sequencing and biomarker reporting were developed.
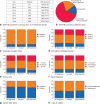
Results: More than 10 000 patients from nine hospitals have currently consented to inclusion in SCAN-B with high (90 per cent) inclusion rates from both university and secondary hospitals. Tumour samples and successful RNA sequencing are being obtained from more than 70 per cent of patients, showing excellent representation compared with the national quality registry as a truly population-based cohort. Molecular biomarker reports can be delivered to multidisciplinary conferences within 1 week.
Conclusion: Population-based collection of fresh tumour tissue is feasible given a decisive joint effort between academia and collaborative healthcare groups, and with governmental support. An infrastructure for genomic analysis and prompt data output paves the way for novel systemic therapy for patients from all hospitals, irrespective of size and location.
© 2018 The Authors. BJS published by John Wiley & Sons Ltd on behalf of BJS Society Ltd.
Figures
References
-
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA et al Molecular portraits of human breast tumours. Nature 2000; 406: 747–52.1. - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) , Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S et al Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient‐level meta‐analysis of randomised trials. Lancet 2011; 378: 771–784. - PMC - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) , Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M et al Comparisons between different polychemotherapy regimens for early breast cancer: meta‐analyses of long‐term outcome among 100 000 women in 123 randomised trials. Lancet 2012; 379: 432–444. - PMC - PubMed
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER‐2/neu oncogene. Science 1987; 235: 177–182. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

