The impact of a dose of the angiotensin receptor blocker valsartan on post-myocardial infarction ventricular remodelling
- PMID: 29341471
- PMCID: PMC5880661
- DOI: 10.1002/ehf2.12249
The impact of a dose of the angiotensin receptor blocker valsartan on post-myocardial infarction ventricular remodelling
Abstract
Aims: Although clinical guidelines advocate the use of the highest tolerated dose of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers after acute myocardial infarction (MI), the optimal dosing or the risk-benefit profile of different doses have not been fully identified.
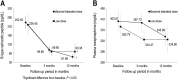
Methods and results: In this multicentre trial, 495 Korean patients with acute ST segment elevation MI and subnormal left ventricular (LV) ejection fraction (<50%) were randomly allocated (2:1) to receive maximal tolerated dose of valsartan (titrated up to 320 mg/day, n = 333) or low-dose valsartan (80 mg/day, n = 162) treatment. The primary objective was to assess the changes in echocardiographic parameters of LV remodelling from baseline to 12 months after discharge. After treatment, end-diastolic LV volume (LVEDV) decreased significantly in the low-dose group, but the difference in LVEDV changes was insignificant between the maximal-tolerated-dose and low-dose groups. End-systolic LV volume decreased significantly in both groups, to a similar degree between groups. LV ejection fraction rose significantly in both study groups, to a similar degree. Changes in plasma levels of neurohormones were also comparable between the two groups. Drug-related adverse effects occurred more frequently in the maximal-tolerated-dose group than in the low-dose group (7.96 vs. 0.69%, P < 0.001).
Conclusions: In the present study, treatment with the maximal tolerated dose of valsartan did not exhibit a superior effect on post-MI LV remodelling compared with low-dose treatment and was associated with a greater frequency of adverse effect in Korean patients. Further study with a sufficient number of cases and statistical power is warranted to verify the findings of the present study.
Keywords: Dose; Myocardial infarction; Valsartan; Ventricular remodelling.
© 2018 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of the European Society of Cardiology.
Figures



References
-
- Domanski MJ, Exner DV, Borkowf CB, Geller NL, Rosenberg Y, Pfeffer MA. Effect of angiotensin converting enzyme inhibition on sudden cardiac death in patients following acute myocardial infarction. A meta‐analysis of randomized clinical trials. J Am Coll Cardiol 1999; 33: 598–604. - PubMed
-
- Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau J, Rouleau JL, Rutherford J, Wertheimer JH, Hawkins CM, on behalf of the SAVE Investigators . Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the Survival and Ventricular Enlargement Trial. N Engl J Med 1992; 327: 669–677. - PubMed
-
- The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators . Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet 1993; 342: 821–828. - PubMed
-
- Kober L, Trop‐Pedersen C, Carisen JE, Bagger H, Eliasen P, Lyngborg K, Videbek J, Cole DS, Auclert L, Pauly N, Aliot E, Persson S, Camm AJ, Trandolapril Cardiac Evaluation (TRACE) Study Group . A clinical trial of the angiotensin‐converting enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 1995; 333: 1670–1676. - PubMed
-
- Pfeffer MA, McMurray JJV, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM, Valsartan in Acute Myocardial Infarction Trial Investigators . Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349: 1893–1906. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
