α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling
- PMID: 29342138
- PMCID: PMC6007875
- DOI: 10.1038/nature25451
α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling
Abstract
The ageing suppressor α-klotho binds to the fibroblast growth factor receptor (FGFR). This commits FGFR to respond to FGF23, a key hormone in the regulation of mineral ion and vitamin D homeostasis. The role and mechanism of this co-receptor are unknown. Here we present the atomic structure of a 1:1:1 ternary complex that consists of the shed extracellular domain of α-klotho, the FGFR1c ligand-binding domain, and FGF23. In this complex, α-klotho simultaneously tethers FGFR1c by its D3 domain and FGF23 by its C-terminal tail, thus implementing FGF23-FGFR1c proximity and conferring stability. Dimerization of the stabilized ternary complexes and receptor activation remain dependent on the binding of heparan sulfate, a mandatory cofactor of paracrine FGF signalling. The structure of α-klotho is incompatible with its purported glycosidase activity. Thus, shed α-klotho functions as an on-demand non-enzymatic scaffold protein that promotes FGF23 signalling.
Conflict of interest statement
O.W.M. has done paid consultation for AbbVie, Allena, Amgen, and Tricida. He also sits on the board of Klotho Therapeutics. All the other authors have no competing financial interests to declare.
Figures




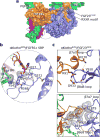








Comment in
-
Ageing-related receptors resolved.Nature. 2018 Jan 25;553(7689):409-410. doi: 10.1038/d41586-017-09032-4. Nature. 2018. PMID: 29368738 No abstract available.
-
Unraveling Endocrine FGF Signaling Complex to Combat Metabolic Diseases.Trends Biochem Sci. 2018 Aug;43(8):563-566. doi: 10.1016/j.tibs.2018.05.001. Epub 2018 Jun 9. Trends Biochem Sci. 2018. PMID: 29895507
References
-
- Schlessinger J, et al. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol Cell. 2000;6:743–750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

