Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases
- PMID: 29342248
- PMCID: PMC5888946
- DOI: 10.1093/annonc/mdx797
Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases
Abstract
Background: This report assesses the efficacy and safety of palbociclib plus endocrine therapy (ET) in women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer (ABC) with or without visceral metastases.
Patients and methods: Pre- and postmenopausal women with disease progression following prior ET (PALOMA-3; N = 521) and postmenopausal women untreated for ABC (PALOMA-2; N = 666) were randomized 2 : 1 to ET (fulvestrant or letrozole, respectively) plus palbociclib or placebo. Progression-free survival (PFS), safety, and patient-reported quality of life (QoL) were evaluated by prior treatment and visceral involvement.
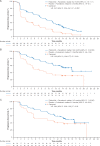
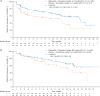
Results: Visceral metastases incidence was higher in patients with prior resistance to ET (58.3%, PALOMA-3) than in patients naive to ET in the ABC setting (48.6%, PALOMA-2). In patients with prior resistance to ET and visceral metastases, median PFS (mPFS) was 9.2 months with palbociclib plus fulvestrant versus 3.4 months with placebo plus fulvestrant [hazard ratio (HR), 0.47; 95% confidence interval (CI), 0.35-0.61], and objective response rate (ORR) was 28.0% versus 6.7%, respectively. In patients with nonvisceral metastases, mPFS was 16.6 versus 7.3 months, HR 0.53; 95% CI 0.36-0.77. In patients with visceral disease and naive to ET in the advanced disease setting, mPFS was 19.3 months with palbociclib plus letrozole versus 12.9 months with placebo plus letrozole (HR 0.63; 95% CI 0.47-0.85); ORR was 55.1% versus 40.0%; in patients with nonvisceral disease, mPFS was not reached with palbociclib plus letrozole versus 16.8 months with placebo plus letrozole (HR 0.50; 95% CI 0.36-0.70). In patients with prior resistance to ET with visceral metastases, palbociclib plus fulvestrant significantly delayed deterioration of QoL versus placebo plus fulvestrant, whereas patient-reported QoL was maintained with palbociclib plus letrozole in patients naive to endocrine-based therapy for ABC.
Conclusions: Palbociclib plus ET prolonged mPFS in patients with visceral metastases, increased ORRs, and in patients previously treated for ABC, delayed QoL deterioration, presenting a standard treatment option among patients with visceral metastases amenable to endocrine-based therapy.
Clinical trial registration: NCT01942135, NCT01740427.
Figures



References
-
- Rugo H, Rumble B, Macrae E. et al. Endocrine therapy for hormone receptor–positive metastatic breast cancer: American Society of Clinical Oncology guideline. JCO 2016; 34: 3069–3103. - PubMed
-
- Cardoso F, Costa A, Senkus E. et al. 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Breast 2017; 31: 244–259. - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer. Version 2.2017. https://www.nccn.org/store/login/login.aspx? ReturnURL=https://www.nccn.... (5 July 2017, date last accessed).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

