A Xylenol Orange-Based Screening Assay for the Substrate Specificity of Flavin-Dependent para-Phenol Oxidases
- PMID: 29342886
- PMCID: PMC6017454
- DOI: 10.3390/molecules23010164
A Xylenol Orange-Based Screening Assay for the Substrate Specificity of Flavin-Dependent para-Phenol Oxidases
Abstract
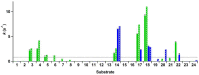
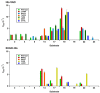
Vanillyl alcohol oxidase (VAO) and eugenol oxidase (EUGO) are flavin-dependent enzymes that catalyse the oxidation of para-substituted phenols. This makes them potentially interesting biocatalysts for the conversion of lignin-derived aromatic monomers to value-added compounds. To facilitate their biocatalytic exploitation, it is important to develop methods by which variants of the enzymes can be rapidly screened for increased activity towards substrates of interest. Here, we present the development of a screening assay for the substrate specificity of para-phenol oxidases based on the detection of hydrogen peroxide using the ferric-xylenol orange complex method. The assay was used to screen the activity of VAO and EUGO towards a set of twenty-four potential substrates. This led to the identification of 4-cyclopentylphenol as a new substrate of VAO and EUGO and 4-cyclohexylphenol as a new substrate of VAO. Screening of a small library of VAO and EUGO active-site variants for alterations in their substrate specificity led to the identification of a VAO variant (T457Q) with increased activity towards vanillyl alcohol (4-hydroxy-3-methoxybenzyl alcohol) and a EUGO variant (V436I) with increased activity towards chavicol (4-allylphenol) and 4-cyclopentylphenol. This assay provides a quick and efficient method to screen the substrate specificity of para-phenol oxidases, facilitating the enzyme engineering of known para-phenol oxidases and the evaluation of the substrate specificity of novel para-phenol oxidases.
Keywords: enzyme kinetics; flavoprotein; oxidase; screening assay; substrate specificity.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures


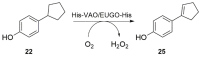

References
-
- De Jong E., Van Berkel W.J.H., Van der Zwan R.P., Bont J.A.M. Purification and characterization of vanillyl-alcohol oxidase from Penicillium simplicissimum: A novel aromatic alcohol oxidase containing covalently bound FAD. Eur. J. Biochem. 1992;208:651–657. doi: 10.1111/j.1432-1033.1992.tb17231.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

