Polymer-Ceramic Composite Scaffolds: The Effect of Hydroxyapatite and β-tri-Calcium Phosphate
- PMID: 29342890
- PMCID: PMC5793627
- DOI: 10.3390/ma11010129
Polymer-Ceramic Composite Scaffolds: The Effect of Hydroxyapatite and β-tri-Calcium Phosphate
Abstract
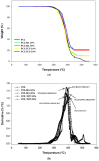
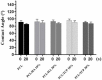
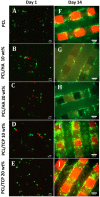
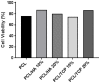
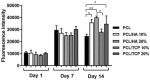
The design of bioactive scaffolds with improved mechanical and biological properties is an important topic of research. This paper investigates the use of polymer-ceramic composite scaffolds for bone tissue engineering. Different ceramic materials (hydroxyapatite (HA) and β-tri-calcium phosphate (TCP)) were mixed with poly-ε-caprolactone (PCL). Scaffolds with different material compositions were produced using an extrusion-based additive manufacturing system. The produced scaffolds were physically and chemically assessed, considering mechanical, wettability, scanning electron microscopy and thermal gravimetric tests. Cell viability, attachment and proliferation tests were performed using human adipose derived stem cells (hADSCs). Results show that scaffolds containing HA present better biological properties and TCP scaffolds present improved mechanical properties. It was also possible to observe that the addition of ceramic particles had no effect on the wettability of the scaffolds.
Keywords: 3D printing; hydroxyapatite; scaffold; tri-calcium phosphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Comparison of 3D-Printed Poly-ɛ-Caprolactone Scaffolds Functionalized with Tricalcium Phosphate, Hydroxyapatite, Bio-Oss, or Decellularized Bone Matrix<sup/>Tissue Eng Part A. 2017 Jun;23(11-12):503-514. doi: 10.1089/ten.TEA.2016.0418. Epub 2017 Feb 7. Tissue Eng Part A. 2017. PMID: 28027692
-
Accelerated Degradation of Poly-ε-caprolactone Composite Scaffolds for Large Bone Defects.Polymers (Basel). 2023 Jan 28;15(3):670. doi: 10.3390/polym15030670. Polymers (Basel). 2023. PMID: 36771970 Free PMC article.
-
Biomimetic composite coating on rapid prototyped scaffolds for bone tissue engineering.Acta Biomater. 2011 Feb;7(2):809-20. doi: 10.1016/j.actbio.2010.09.010. Epub 2010 Sep 16. Acta Biomater. 2011. PMID: 20849985
-
Solvent-dependent properties of electrospun fibrous composites for bone tissue regeneration.Acta Biomater. 2010 Jan;6(1):90-101. doi: 10.1016/j.actbio.2009.07.028. Epub 2009 Jul 23. Acta Biomater. 2010. PMID: 19631769
-
Review on vat photopolymerization additive manufacturing of bioactive ceramic bone scaffolds.J Mater Chem B. 2023 Oct 18;11(40):9572-9596. doi: 10.1039/d3tb01236k. J Mater Chem B. 2023. PMID: 37727909 Review.
Cited by
-
Effect of Electron Beam Sterilization on Three-Dimensional-Printed Polycaprolactone/Beta-Tricalcium Phosphate Scaffolds for Bone Tissue Engineering.Tissue Eng Part A. 2019 Feb;25(3-4):248-256. doi: 10.1089/ten.TEA.2018.0130. Epub 2018 Oct 27. Tissue Eng Part A. 2019. PMID: 30234441 Free PMC article.
-
A 3D Printed Composite Scaffold Loaded with Clodronate to Regenerate Osteoporotic Bone: In Vitro Characterization.Polymers (Basel). 2021 Jan 1;13(1):150. doi: 10.3390/polym13010150. Polymers (Basel). 2021. PMID: 33401469 Free PMC article.
-
Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration.Polymers (Basel). 2020 Dec 1;12(12):2881. doi: 10.3390/polym12122881. Polymers (Basel). 2020. PMID: 33271770 Free PMC article. Review.
-
Tough magnesium phosphate-based 3D-printed implants induce bone regeneration in an equine defect model.Biomaterials. 2020 Dec;261:120302. doi: 10.1016/j.biomaterials.2020.120302. Epub 2020 Aug 23. Biomaterials. 2020. PMID: 32932172 Free PMC article.
-
Biochemical, toxicological, and microbiological assessment of calcined poultry manure for potential use as bone scaffold material.Heliyon. 2024 Sep 24;10(19):e38378. doi: 10.1016/j.heliyon.2024.e38378. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39391474 Free PMC article.
References
-
- Al-Tamimi A.A., Fernandes P.R.A., Peach C., Cooper G., Diver C., Bartolo P.J. Metallic bone fixation implants: A novel design approach for reducing the stress shielding phenomenon. Virtual Phys. Prototyp. 2017;12:141–151. doi: 10.1080/17452759.2017.1307769. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

