Role of Aquaporins in Determining Carbon and Nitrogen Status in Higher Plants
- PMID: 29342938
- PMCID: PMC5795985
- DOI: 10.3390/ijms19010035
Role of Aquaporins in Determining Carbon and Nitrogen Status in Higher Plants
Abstract
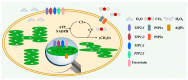
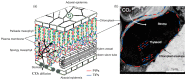
Aquaporins (AQPs) are integral membrane proteins facilitating the transport of water and some small neutral molecules across cell membranes. In past years, much effort has been made to reveal the location of AQPs as well as their function in water transport, photosynthetic processes, and stress responses in higher plants. In the present review, we paid attention to the character of AQPs in determining carbon and nitrogen status. The role of AQPs during photosynthesis is characterized as its function in transporting water and CO₂ across the membrane of chloroplast and thylakoid; recalculated results from published studies showed that over-expression of AQPs contributed to 25% and 50% increases in stomatal conductance (gs) and mesophyll conductance (gm), respectively. The nitrogen status in plants is regulated by AQPs through their effect on water flow as well as urea and NH₄⁺ uptake, and the potential role of AQPs in alleviating ammonium toxicity is discussed. At the same time, root and/or shoot AQP expression is quite dependent on both N supply amounts and forms. Future research directions concerning the function of AQPs in regulating plant carbon and nitrogen status as well as C/N balance are also highlighted.
Keywords: aquaporins; carbon; nitrogen; transport; uptake.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Role of aquaporins in leaf physiology.J Exp Bot. 2009;60(11):2971-85. doi: 10.1093/jxb/erp171. Epub 2009 Jun 19. J Exp Bot. 2009. PMID: 19542196 Review.
-
Aquaporins as a link between water relations and photosynthetic pathway in abiotic stress tolerance in plants.Gene. 2019 Mar 1;687:166-172. doi: 10.1016/j.gene.2018.11.031. Epub 2018 Nov 14. Gene. 2019. PMID: 30445023 Review.
-
Plant aquaporins: A frontward to make crop plants drought resistant.Physiol Plant. 2021 Jun;172(2):1089-1105. doi: 10.1111/ppl.13416. Epub 2021 Apr 20. Physiol Plant. 2021. PMID: 33826759 Review.
-
Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: crop water-use efficiency, growth and yield.Plant Cell Environ. 2015 Sep;38(9):1785-93. doi: 10.1111/pce.12410. Epub 2014 Aug 16. Plant Cell Environ. 2015. PMID: 25039365 Review.
-
Carbon dioxide and water transport through plant aquaporins.Plant Cell Environ. 2017 Jun;40(6):938-961. doi: 10.1111/pce.12844. Epub 2016 Dec 1. Plant Cell Environ. 2017. PMID: 27739588 Review.
Cited by
-
Melatonin Mitigates Chilling-Induced Oxidative Stress and Photosynthesis Inhibition in Tomato Plants.Antioxidants (Basel). 2020 Mar 6;9(3):218. doi: 10.3390/antiox9030218. Antioxidants (Basel). 2020. PMID: 32155702 Free PMC article.
-
Role of Microbes in Alleviating Crop Drought Stress: A Review.Plants (Basel). 2024 Jan 27;13(3):384. doi: 10.3390/plants13030384. Plants (Basel). 2024. PMID: 38337917 Free PMC article. Review.
-
Enhanced Stomatal Conductance Supports Photosynthesis in Wheat to Improved NH4+ Tolerance.Plants (Basel). 2023 Dec 27;13(1):86. doi: 10.3390/plants13010086. Plants (Basel). 2023. PMID: 38202394 Free PMC article.
-
Knockout of SlSBPASE Suppresses Carbon Assimilation and Alters Nitrogen Metabolism in Tomato Plants.Int J Mol Sci. 2018 Dec 14;19(12):4046. doi: 10.3390/ijms19124046. Int J Mol Sci. 2018. PMID: 30558146 Free PMC article.
-
The Arabidopsis PIP1;1 Aquaporin Represses Lateral Root Development and Nitrate Uptake Under Low Nitrate Availability.Plant Cell Environ. 2025 Feb;48(2):1500-1513. doi: 10.1111/pce.15222. Epub 2024 Oct 27. Plant Cell Environ. 2025. PMID: 39462913 Free PMC article.
References
-
- Denker B.M., Smith B.L., Kuhajda F.P., Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 1988;263:15634–15642. - PubMed
-
- Maurel C., Javot H., Lauvergeat V., Gerbeau P., Tournaire C., Santoni V., Heyes J. Molecular physiology of aquaporins in plants. Int. Rev. Cytol. Surv. Cell Biol. 2002;215:105–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

