Barriers to timely diagnosis of interstitial lung disease in the real world: the INTENSITY survey
- PMID: 29343236
- PMCID: PMC5773175
- DOI: 10.1186/s12890-017-0560-x
Barriers to timely diagnosis of interstitial lung disease in the real world: the INTENSITY survey
Abstract
Background: The diagnosis of idiopathic pulmonary fibrosis (IPF) and other interstitial lung diseases (ILD) presents significant clinical challenges. To gain insights regarding the diagnostic experience of patients with ILD and to identify potential barriers to a timely and accurate diagnosis, we developed an online questionnaire and conducted a national survey of adults with a self-reported diagnosis of ILD.
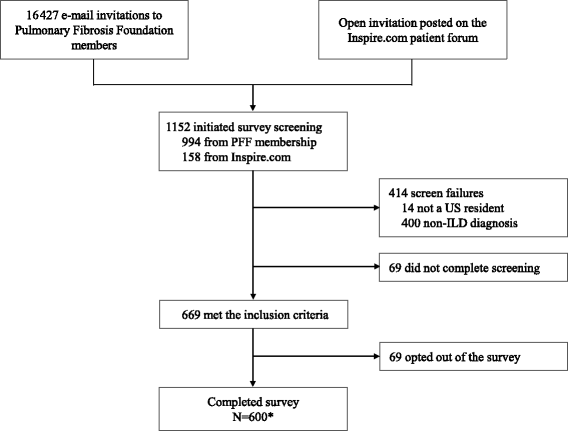
Methods: A pre-specified total of 600 subjects were recruited to participate in a 40-question online survey. E-mail invitations containing a link to the survey were sent to 16 427 registered members of the Pulmonary Fibrosis Foundation. Additionally, an open invitation was posted on an online forum for patients and caregivers ( www.inspire.com ). The recruitment and screening period was closed once the pre-defined target number of respondents was reached. Eligible participants were adult U.S. residents with a diagnosis of IPF or a non-IPF ILD.
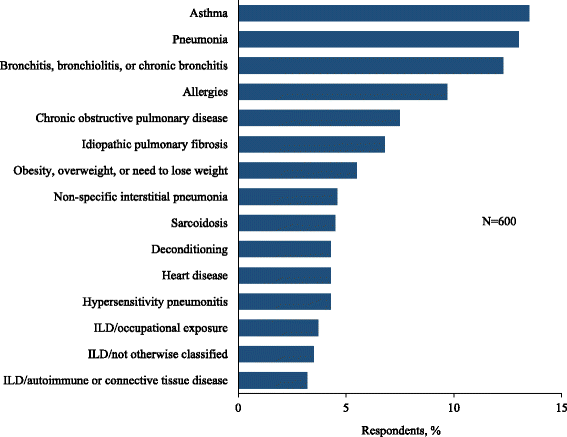
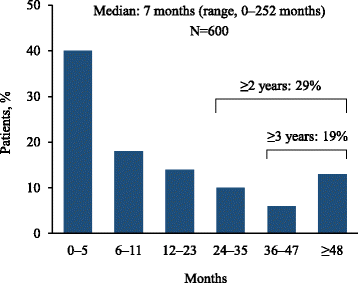
Results: A total of 600 eligible respondents met the eligibility criteria and completed the survey. Of these, 55% reported ≥ 1 misdiagnosis and 38% reported ≥ 2 misdiagnoses prior to the current diagnosis. The most common misdiagnoses were asthma (13.5%), pneumonia (13.0%), and bronchitis (12.3%). The median time from symptom onset to current diagnosis was 7 months (range, 0-252 months), with 43% of respondents reporting a delay of ≥ 1 year and 19% reporting a delay of ≥ 3 years. Sixty-one percent of respondents underwent at least one invasive diagnostic procedure.
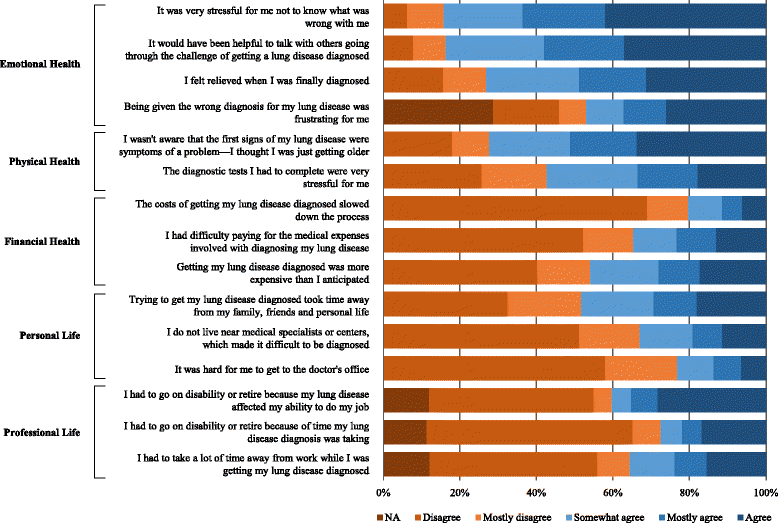
Conclusions: While a minority of patients with ILD will experience an appropriate and expedient diagnosis, the more typical diagnostic experience for individuals with ILD is characterized by considerable delays, frequent misdiagnosis, exposure to costly and invasive diagnostic procedures, and substantial use of healthcare resources. These findings suggest a need for physician education, development of clinical practice recommendations, and improved diagnostic tools aimed at improving diagnostic accuracy in patients with ILD.
Keywords: Diagnosis; Idiopathic pulmonary fibrosis; Interstitial lung disease.
Conflict of interest statement
Ethics approval and consent to participate
The research would be considered exempt under applicable federal regulations (45 CFR 46.101b) based on the American Association for Public Opinion Research policy participant category. Our respondents were active participants in that they opted-in to participate in the research based on their choice to continue from the screener to the survey. Moreover, our respondents were informed that their participation “gives you an opportunity to share your experiences when being diagnosed with PF”.
Consent for publication
Not applicable.
Competing interests
GPC has served as an investigator or collaborator in industry-sponsored clinical trials (Boehringer Ingelheim, Bristol Myers Squibb, Fibrogen, Genentech, Gilead Sciences, Global Blood Therapeutics, and Intermune) and as an advisor to Boehringer Ingelheim, InterMune, Genentech, and Global Blood Therapeutics; he currently serves as the Chief Medical Officer of the Pulmonary Fibrosis Foundation. PB is an employee of Veracyte. SD reports no competing interests. DJL served as a consultant for Boehringer Ingelheim, Genentech, Gilead Sciences, Galapagos, Patara, Phillips Respironics, the Pulmonary Fibrosis Foundation, and Veracyte, and has received institutional research grants from Bayer, Boehringer Ingelheim, Fibrogen, Gilead Sciences, and Global Blood Therapeutics.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

